The present paper describes the role of Mn on the properties of a Cu- Al-Ni-Zn shape memory alloy. The effect of addition of different amounts of Mn has been studied on a Cu-12Al-4Ni-10Zn alloy, known to exhibit shape memory properties. The transformation temperatures, phase precipitation and thermal properties have been determined. It has been found that the quantity of Mn has a significant effect on the formation, morphology, and structure of the obtained martensite. Therefore, the properties of these alloys are varied in accordance of these effects. The efficacy of adding Mn and its quantity has been described in this study and a correlation is attempted to relate it to the thermal properties.
Properties attained in shape memory alloys are very much dependant on the number of factors including exact chemical composition, heat treatment and processing conditions. Reports suggest that, slight modification of composition and or alloy addition can drastically change the transformation temperatures by over a range of temperature [1-6]. Keeping this in mind and with the constant demand of achieving high transformation temperatures, researchers, worldwide are attempting to alter composition and play with the heat treatment cycles to achieve this demand. A few alloy compositions have shown potential in this respect and recent studies [7-9] have reported a drastic increase in the transformation temperatures, with minor alloying additions and/or variations in alloying constituents. An attempt has been made in the present paper to understand the effect of adding different quantities of Mn to a Cu-12Al-4Ni-10Zn alloy, that has been reported to exhibit good shape memory properties, mechanical properties including ductility and relatively high transformation temperatures [9- 12]. The transformation temperatures, phase precipitation and thermal properties have been determined for five compositions of the alloy with varying Mn content between 5 to 10 wt.%. The efficacy of adding Mn and its quantity has been described in the study and a correlation is attempted to relate it to the thermal properties.
Cu-12Al-4Ni-10Zn alloy was used as the base alloy and different quantities of Mn, varying from 5 to 10 wt.% of Mn were added to make in total five compositions, using liquid metallurgy route, 99.9% purity metals of copper, aluminium, manganese and the respective quaternary alloying elements by taking in right quantities to weigh 1000 g of the alloy in total and melted together. The chemical composition of the proposed alloys and its designations are given in Table 1.
Samples from the cast alloys were subject to o homogenisation treatment, which was optimised at 200 oC for 2 hrs in a muffle furnace, and the furnace was cooled to remove inhomogenity of casting. These were then quenched into iced water after holding samples for 2 hrs at 920 ºC. In a parallel study, the quenching conditions were optimised for the alloys and the present paper reports the structure and properties of the optimised conditions, only as determined by the microstructure of the quenched samples.
The homogenised and quenched samples were polished metallographically using an automatic polishing machine capable of polishing six samples together (Buehler make, Model EcoMet 3000) using standard procedures. The microstructure was observed in an optical microscope (LEICA make, Model LEICA DM 6000 M and Metalloplan) and in FESEM (Field Emission Scanning Electron Microscope) (FEI make, Model Nova Nano SEM 430) were optical micrographs, which were not properly resolved.
X-ray Diffraction studies were carried out on the quenched samples (Bruker make, Model D8 Advanced) from 2θ of 20 to 80, at a speed of 0.01o 2θ/s using CuKα target; and the phases were identified.
Differential Scanning Calorimetric (DSC) studies were carried out on quenched samples using a Differential Scanning Calorimeter (Mettler Toledo make, Model DSC1STARe SYSTEM). Powder samples scrapped from the quenched samples of less than 40 mg weight was used for the tests. Tests were carried out from room temperature to 600 oC maintaining a constant rate of 10o C/min. The change in enthalpy and entropy was calculated from the DSC peaks.
The hardness of samples was determined using Hardness Tester adopting Vickers scale with a load of 5 kgf.
All the above tests were carried out to understand the difference, if any, in the microstructure and shape memory property possibilities in the alloys as a result of varying Mn, and with an aim to optimise its content. The X-ray diffraction and metallographic studies help in identifying desired phase formation and from the DSC plots, the energy absorbed can be known in addition to the transformation temperatures. The idea of alloying is to increase/play with the transformation temperatures, a desired property in shape memory alloys. Further, from the energy changes calculated, an attempt has been made to relate properties attained to the thermal changes observed.
The change in entropy and enthalpy is calculated from the DSC data [13, 14], DSC plots heat flow [W], as a function of temperature (degree centigrade). The integral of the transformation peak in cooling and heating yields the change in enthalpy as a function of temperature. The plot of heat flow (w/g) per weight is converted as a function of temperature. This is then multiplied by the peak width and divided by the heating/cooling rate to calculate the change in enthalpy (J/g). This is done for the heating and cooling cycles and the average values are calculated, as follows,
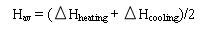
The change in entropy [ΔS], on the other hand, can be determined by dividing the average value of the enthalpy with equilibrium temperature,
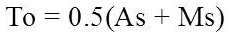
where, As is Austenite start temperature and Ms is Martensite start temperature.

A measure of the enthalpy and entropy change gives the thermal properties of the alloy.
The alloys under study, its designation and its chemical composition are analysed in Table 1.
The structure of the base alloy [Sample A in Figure 1] show grains and precipitation in the grains. The average grain size is 80-100 µm. The same structure is maintained in other alloys (samples B, C, D and E), however, there is a variation in the grain size with composition between 50-100 µm. This type of α+β granular structure is an indication of possible shape memory properties [5,9].
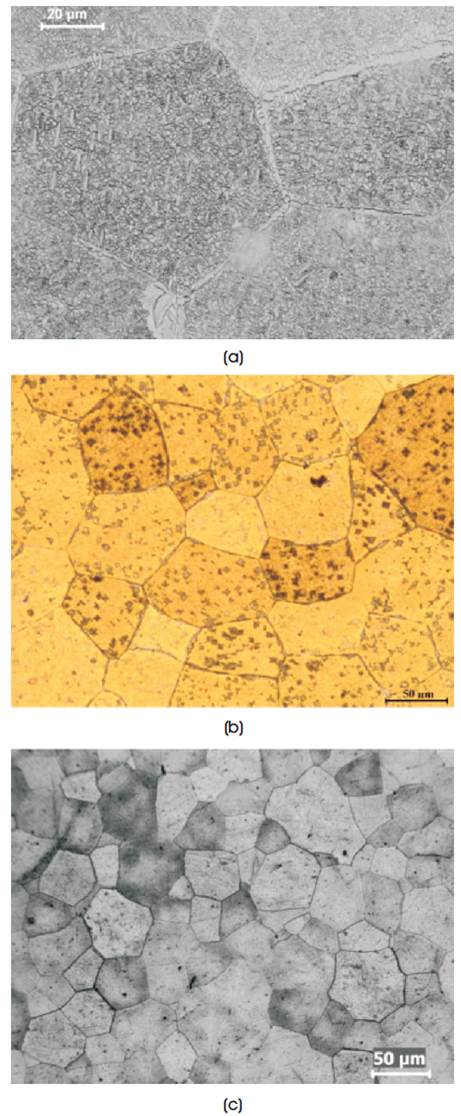
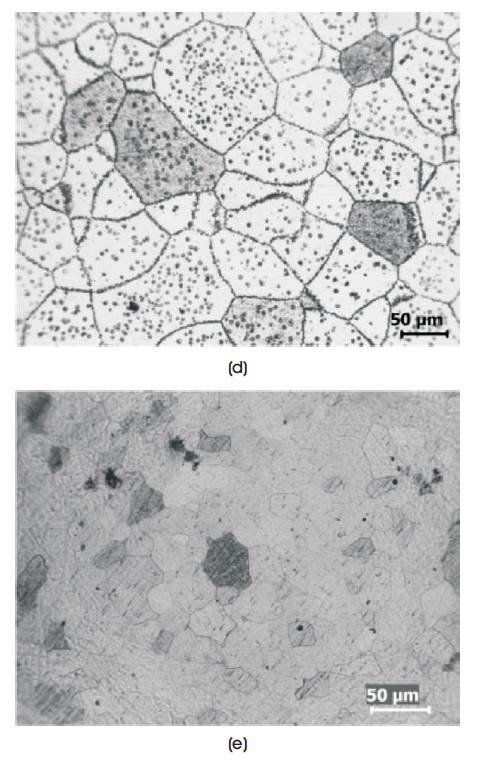
Figure 1. Microstructure of Homogenised Alloys
The microstructure of quenched samples at low and high magnification is shown in Figure 2. Quenching results in martensite formation within the grains for the base alloy, (Figure 2a) with a considerable decrease in grain size, as compared to the homogenised condition. The martensite formation though marked in a higher magnification micrograph (Figure 2b) is not the plate- or spear-like martensite, which is the most commonly observed microstructures in the quenched Cu-Zn-Al shape memory alloy observed. Instead the martensite phase shows different patterns mostly flower shaped typical of γ-phase is observed [15].
It is interesting to note that, though the precipitation of martensite is marked in the base alloy, addition of Mn decreases this precipitation and in samples containing upto 7 Mn, there is no clear precipitation of this phase is observed even in FESEM samples, at high magnifications (Figures 2 b & c). However, the phase reappears very lightly within grains, in samples with higher Mn content (Figures 2 d & e).
The effect of addition of Mn to the base alloy is clearly reflected in the hardness. There is a remarkable increase in the hardness over the base alloy for all compositions containing Mn; the increase is directly proportional to the amount of Mn (Figure 3). Homogenisation actually relates to stabilizing the hardness, however even the cast alloys has not shown much variation as can be seen in standard deviation marked in black in Figure 3a. Interestingly, the homogenised samples do not record a fall in hardness as normally expected. Quenching decreases the hardness in general for samples containing Mn however no much difference in hardness is observed on quenching the base alloy [sample A] as shown in Figure 3b. It is reported [16, 17] that, the martensitic phase in Cu-based alloys are softer as unlike for ferrous alloys and a drop in hardness on quenching is an indirect confirmation of the desired martensitic phase formation, that would lead to shape memory properties. Again since the drop in hardness is significant in Sample B, D and E, it is expected that, these alloys will exhibit better shape memory properties due to formation of more martensite.
The XRD pattern of the quenched samples are shown in Figure 4 with the peaks marked. Table 2 lists the main peaks observed. It is seen that, Sample C do not record any peak other than that of Cu/Cu-Zn; the other samples record different peaks. The observed peaks have been identified using JCPDS (Joint Committee on Powder Diffraction Standards) files. The main phase is Cu in all the samples; in addition, precipitation of martensitic phase Cu-Zn has been observed in all the samples. Further, another martensitic phase AlCu has been precipitated in Samples A and D. Micrographs have also shown martensitic phases in quenched samples.
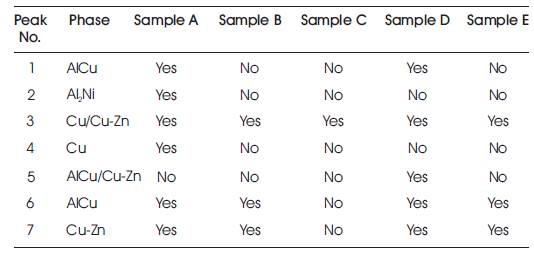
Table 2. Identified XRD Peaks in Quenched Samples
The heating and cooling cycles of the samples heated at room temperature to 600 ºC, and cooling it in the same range maintaining a constant heating/cooling rate of 10 ºC/min are plotted on a comparative basis in Figure 5a. The peaks obtained correspond to the phase transformations. It shows that, clear transformation peaks are observed for all the samples; however the peaks obtained for sample A, B and E do not conform to the martensite-austenite phase transformation. This can be said from the nature of the peak, which is very sharp and also from the high temperatures of the transformation. The samples C and D give clear transformation temperatures for the required martensite-austenite transformation in a range expected of these alloys. It may be noted that, adding upto 8 wt.% Mn increases the transformation temperature range markedly (Figure 5b) that has been reported earlier [9-11].
The effect of adding Mn in appropriate amount can thus be said to be essential for these class of alloys, to exhibit shape memory behaviour, as has been judged from the DSC studies. The amount of Mn added, if between 7 to 8, do not have much effect on the transformation temperatures; however, increasing it to 10 or decreasing it to 5 wt.% results in no proper peaks being recorded for the required transformations.
For the alloys under study, the change in enthalpy and entropy are given in Table 3 for samples, that have exhibited the requisite transformation from martensite to austenite on cooling, and vice versa on heating. The change in enthalpy and entropy values is found to be a function of the composition; the base alloy exhibits the maximum change and addition of Ti, the minimum change. Addition of Cr has the minimum effect on change from the base alloy. These results are coherent with the data given in the literature [13,14].
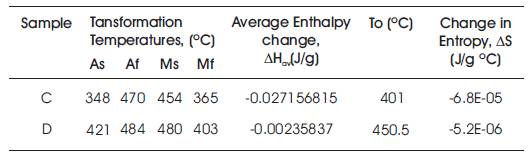
Table 3. Change in Enthalpy and Entropy
The effect of alloying constituent of Mn and its wt.% in a Cu- Al-Ni shape memory alloy has been established by the present study. All the compositions show the requisite α+β phase in the cast structure for shape memory properties; however on quenching, the desired martensite phase is formed only in the base alloy and the alloys with more than 5 wt.% Mn. The XRD data also reiterate the same findings where, martensitic phase is seen to be precipitated in samples A, C and D mainly. Alloys with 10% Mn and less than 5% do not exhibit the required martensite to austenite transformations in DSC studies. This study goes to reiterate the importance of a correct selection of alloying additions for exhibiting structures favouring shape memory behaviour. The transformation temperatures attained in the present study are relatively high, making these alloys with 7- 8 wt.% Mn ideal candidates for high temperature shape memory alloys.
The authors are thankful to CSIR, New Delhi for sponsoring the project on 'Design and Development of Thermo Responsive & Magnetic Shape Memory Materials and th Devices for Engineering Applications' under its 12 Five Year Plan to CSIR, AMPRI Bhopal, under which the related activities were carried out; a part of the findings have been reported in the present paper. Project Fellows are thankful to CSIR, New Delhi for granting their fellowship under the project. Authors acknowledge with gratitude the contribution of the Metallurgical Engineering Department, IIT Chennai, for extending their facilities.