Polymer electrolytes based on ammonium fluoride (NH4F) and poly (vinylidene fluoride-co-hexafluoropropylene) (PVdF- HFP) have been prepared by solution casting technique using Tetrahydrofuran (THF) as a solvent and characterized by complex impedance spectroscopy. Maximum conductivity of 2.17x10-7 S/cm at room temperature has been obtained for polymer electrolytes PVdF-HFP+10wt%NH4F. The effect of different plasticizers Propylene Carbonate (PC), Dimethylformamide (DMF) and Dimethylacetamide (DMA) on conductivity behavior has been studied. It has been found that, the conductivity of polymer electrolytes increases by three orders of magnitude from 10-7 to 10-4 S/cm with the addition of plasticizers. Maximum room temperature conductivity of 8.8x10-6 S/cm, 7.8x10-5 S/cm and 1.1x10- 4 S/cm has been observed for plasticized polymer electrolytes containing PVdF-HFP+30 wt% NH4F and 95 wt% PC, DMF, DMA respectively, which can be explained on the basis of donor numbers of plasticizers used. The addition of plasticizer to polymer electrolytes has been observed to decrease in glass transition temperature (Tg ). The variation of conductivity with temperature suggests that, these polymer electrolytes are thermally stable and small change in conductivity with temperature is suitable for their use in practical applications like solid state batteries, fuel cells, electro chromic devices, super capacitors, etc.
Polymer electrolytes are found to possess a good ionic exchange property that has led to interesting applications in many solid state ionic devices like high-energy density batteries, fuel cells, sensors, electrochemical display devices, infrared electrochromic devices, etc. [1]-[4]. These electrolytes have a number of advantages over the conventional liquid electrolytes, such as freedom from corrosive liquid leakage, easy fabrication in a wide variety of shapes, ability to form proper electrode-electrolyte contacts, chemical stability etc. [5]-[9]. Polymer electrolytes possess low ionic conductivity at room temperature, which limits their use in practical applications. Many attempts have been made to increase their conductivity, which includes the use of salt with large anions, addition of plasticizers, addition of inert insulating matrix of micro and nano size [10]-[19]. Most of the studies on polymer electrolytes have been reported for plasticized polymer electrolytes in which, the addition of plasticizers enhances the amorphous content which is reported to be the high conducting phase along with lowering of Glass Transition Temperature (Tg).
In the present paper, polymer electrolytes of poly(vinylidene fluoride-co-hexafluoro propylene) (PVdFHFP) and ammonium fluoride (NH4F) has been studied. The effect of donor number of plasticizers on conductivity behaviour of plasticized polymer electrolytes has been studied. The variation of Glass Transition Temperature (Tg) of plasticized polymer electrolytes as a function of plasticizer and the variation of conductivity of polymer electrolytes as a function of temperature has also been studied.
Poly(vinylidenefluoride–co–hexafluoropropylene) (PVdFHFP) (Fluka, Mw = 1,30,000), Ammonium fluoride (NH4F) (Reidel), Tetrahydrofuran (THF) (Merck), Propylene Carbonate (PC) (Lancaster), Dimethylformamide (DMF) (Merck) and Dimethylacetamide (DMA) (Merck) has been used as the starting materials. Polymer electrolytes in the film form were prepared by solution casting technique, using THF as a solvent. Ammonium fluoride was dissolved in THF in stoichiometric quantities and then, PVdF-HFP was added to it and the mixture was stirred to obtain a homogenous solution. The homogenous solution, thus obtained was then poured into polypropylene dishes and the solvent was allowed to evaporate and free standing polymer electrolyte films were obtained. These films were used for various experimental studies. The conductivity was measured by complex impedance spectroscopy with HP4284A precision LCR meter operating in the 20Hz – 1MHz frequency range using a sample holder with silver/platinum electrodes [20]-[24].
The variation of conductivity of polymer electrolytes as a function of salt concentration is shown in Figure 1. From Figure 1, it has been observed that conductivity increases with an increase in salt concentration, reaches a saturation value and even a small decrease in conductivity is observed at higher salt concentrations. Maximum conductivity of 2.17x10-7 S/cm at room temperature has been obtained for polymer electrolytes containing 10 wt% NH4F. At low salt concentrations, the conductivity increases linearly and salt is assumed to be completely dissociated and nearly all ions are available for conduction. With further increase in salt concentration, the conductivity shows a deviation from linear behaviour, reaches a maximum value and then shows a small decrease with the addition of salt. The deviation in linearity of the plot at medium salt concentration is explained to be due to the formation of ion aggregates, which do not take part in the conduction process. At higher salt concentrations, the formation of ion aggregates increases and conductivity attains a saturation value. But with the addition of salt, the viscosity of polymer electrolytes also increases, which reduces the mobility and hence conductivity decreases [9], [13]-[14].
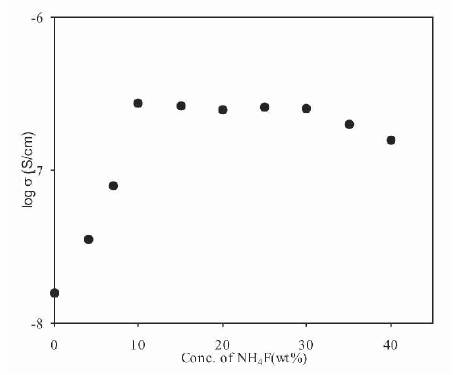
Figure 1. Variation of Conductivity for (PVdF-HFP) –NH4F Polymer Electrolytes with Salt Concentration
The presence of ion aggregates at higher salt concentrations can be checked on the basis of 'mass action consideration' [9], [25]. Figure 2 shows the variation of log σ and log C for (PVdF-HFP)+xNH4F polymer electrolytes. In this Figure, a linear behaviour is observed at low salt concentrations, which suggests that ion aggregates are not present in this concentration range. But at higher salt concentration, a deviation from the linear behaviour is observed, which suggests that, ion aggregate formation takes place and these results are in good agreement with the results of conductivity variation with salt concentration as given in Figure 1. The maximum conductivity of 2.17 10-7 S/cm has been observed for these polymer electrolytes, which is very small for their use in practical applications. To increase the conductivity of these electrolytes, different plasticizers have been used.
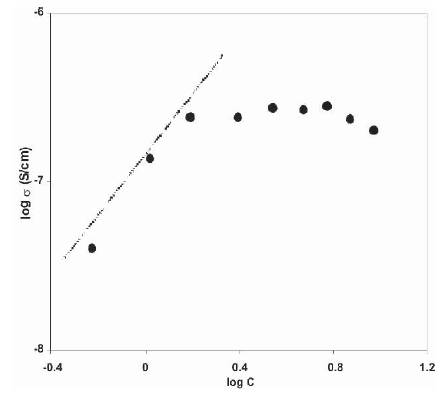
Figure 2. Variation of log σ with log C for (PVdF-HFP) - NH4F Polymer Electrolytes
The conductivity of (PVdF-HFP)+NH4F polymer electrolytes containing 30 wt% NH4F, plasticized with different plasticizers having donor numbers (DMA (27.8)>DMF (26.6)>PC(15.1)) was measured as a function of plasticizer concentration (expressed as wt% of PVdF-HFP). The variation of conductivity of polymer electrolytes at room temperature as a function of the concentration of Propylene Carbonate (PC), Dimethylformamide (DMF) and Dimethylacetamide (DMA) are shown in Figures 3, 4 and 5 respectively.
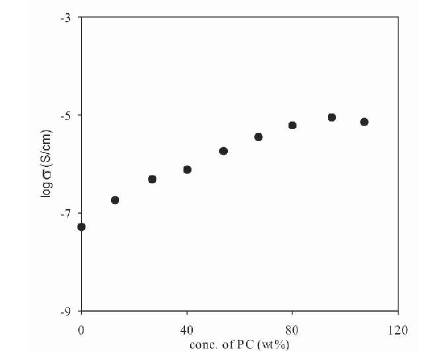
Figure 3. Dependence of Conductivity of Plasticized Polymer Electrolytes (PVdF-HFP+30wt%NH4F+xPC) on PC Concentration
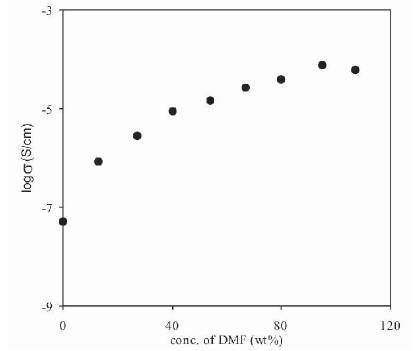
Figure 4. Dependence of Conductivity of Plasticized Polymer Electrolytes (PVdF-HFP+30wt%NH4F+xDMF) on DMF Concentration
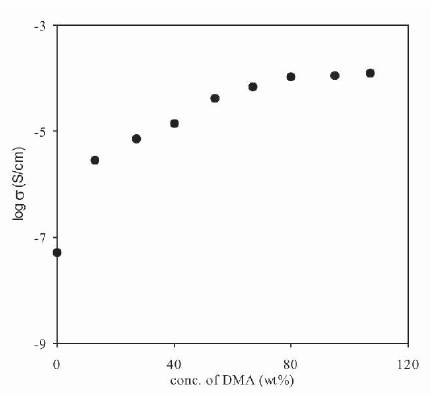
Figure 5. Dependence of Conductivity of Plasticized Polymer Electrolytes (PVdF-HFP+30wt%NH4F+xDMA) on DMA Concentration
From these Figures, it has been observed that the conductivity of plasticized polymer electrolytes is higher than that of the unplasticized polymer electrolytes for all the three plasticizers (PC, DMF, DMA). The increase in conductivity with the addition of plasticizer can be explained on the fact that, the addition of plasticizer leads to an increase in the overall dielectric constant of electrolyte along with an increase in amorphous content [26]-[28]. The conductivity increases by a large amount with the initial addition of a small amount of the plasticizer and then attains a near saturation value at higher concentrations of plasticizer. The saturation in the conductivity occurs because, initially plasticizer increases amorphous content and dissociate ion aggregates / undissociated salt due to the higher dielectric constant. Once the electrolyte becomes amorphous or ion aggregates are dissociated, then further addition of plasticizer shall not have the same effect. The conductivity has been found to increase by nearly three orders of magnitude with the addition of different plasticizers.
The increase in conductivity with the addition of different plasticizers was compared, by studying the variation of conductivity as a function of plasticizer concentration for different plasticizers (PC, DMF, DMA), and the results are shown in Figure 6. Although the conductivity shows a similar increase with the addition of different plasticizers, but the rate of increase in conductivity is different in each case. From Figure 6, it has been observed that, the increase in conductivity with the addition of same amount of different plasticizers to polymer electrolytes is in the following order: σ(DMA)>σ(DMF)>σ(PC) as D.N. (DMA=27.8)>D.N. (DMF=26.6)>D.N.(PC=15.1) [26]-[27]. It has been observed that, as the value of donor number of plasticizer increases, its effect in enhancement of room temperature conductivity also increases.
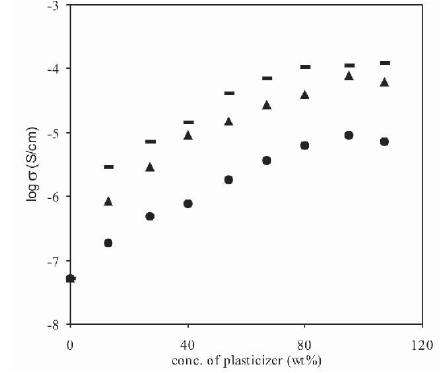
Figure 6. Conductivity Dependence of Polymer Electrolytes (PVdF-HFP+30wt%NH4F) plasticized with PC(•), DMF( ) and DMA(-) with Plasticizer Concentration
) and DMA(-) with Plasticizer Concentration
Maximum room temperature conductivity of 8.8x10-6 S/cm, 7.8x10-5 S/cm and 1.1x10-4 S/cm has been observed for plasticized polymer electrolytes containing PVdF-HFP+ 30wt%NH4F and 95wt% PC, DMF, DMA respectively. As the salt concentration in the electrolyte is kept constant (30 wt%), initially undissociated salt/ion aggregates gets dissociated, but with an increase in concentration of DMA, the amount of undissociated salt/ion aggregates decreases and hence the rate of increase in conductivity is not same, and shows a saturation value at higher salt concentrations.
The conductivity of polymer electrolytes containing 30 wt% NH4F and plasticized with different plasticizers has been measured as a function of temperature. The variation of log conductivity of plasticized polymer electrolytes containing different plasticizers (PC, DMF, DMA) with reciprocal temperature has been given in Figure 7. For comparison, variation of conductivity for unplasticized polymer electrolytes with temperature has also been included in this figure. From Figure 7, it has been observed that the plot for polymer electrolytes are curved in nature, which suggests the amorphous nature of polymer electrolytes containing different plasticizers. It has also been observed that, the conductivity of polymer electrolytes increases with an increase in temperature and conductivity of plasticized polymer electrolytes is more than unplasticized polymer electrolytes at all temperatures. The change in conductivity with temperature over the 25- 100 oC temperature range is very small, which is suitable for practical applications of these plasticized electrolytes in various devices.
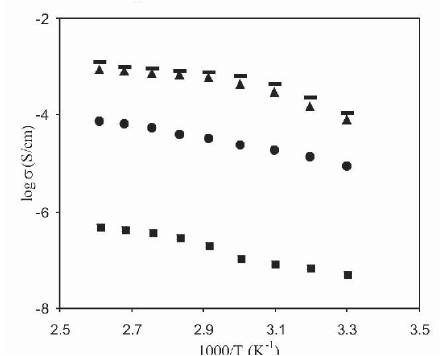
Figure 7. The Variation of Conductivity with Temperature for Unplasticized ( ) and Plasticized Polymer Electrolytes (PVdF-HFP)+30wt% NH4F with 95wt% PC (•), DMF (
) and Plasticized Polymer Electrolytes (PVdF-HFP)+30wt% NH4F with 95wt% PC (•), DMF ( ) and DMA (-).
) and DMA (-).
The addition of plasticizer to polymer electrolytes has been observed to decrease in glass transition temperature (Tg ) [8- 10, 29]. The 'Tg ' of plasticized polymer electrolytes containing different amount of DMA (PVdF-HFP+30wt% NH4F+xwt% DMA) was measured and its variation as a function of DMA concentration is shown in Figure 8. From Figure 8, it has been observed that 'Tg ' of the electrolytes decreases with the addition of DMA, which could be due to an increase in the chain flexibility [29], with which the conductivity increases.
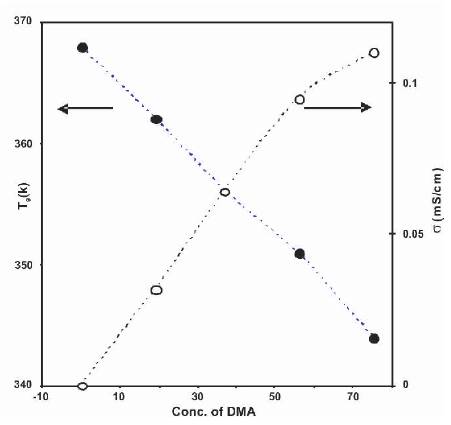
Figure 8. Dependence of Glass Transition Temperature (Tg ) (•) and Conductivity (o) on DMA Concentration (wt% of PVdF-HFP) for Polymer Electrolytes (PVdF-HFP)+30wt% NH4F
Maximum ionic conductivity of 2.17x10-7 S/cm at room temperature has been observed for unplasticized polymer electrolytes containing 10 wt% NH4F. Salt is fully dissociated at low concentration and ion aggregates are present at higher concentrations of salt. The conductivity of unplasticized polymer electrolyte has been observed to increase by nearly three orders of magnitude from 10- 7–10-4 S/cm with the addition of plasticizers. The large increase in conductivity has been explained to be due to the dissociation of undissociated salt/ion aggregates present in the polymer electrolytes with the addition of plasticizer (DMA). Maximum room temperature conductivity of 8.8x10-6 S/cm, 7.8x10-5 S/cm and 1.1x10- 4 S/cm has been observed for plasticized polymer electrolytes containing PVdF-HFP+30wt% NH4F and 95wt% PC, DMF, DMA respectively. The addition of plasticizer to polymer electrolytes has been observed to decrease in Glass Transition Temperature (Tg). The variation of conductivity with temperature shows a small change, which is suitable for their use in practical applications like solid state batteries, fuel cells, electrochromic devices, super capacitors, etc.
The authors are thankful to the Department of Physics, Guru Nanak Dev University, Amritsar, India for experimental studies and University Grants Commission, New Delhi for financial support in the form of Research Project No. 8- 2(172)/2011(MRP/NRCB).