 |
i-manager's Journal on Future Engineering and Technology |
View PDF |
|||
| Volume :11 | No :2 | Issue :-2016 | Pages :37-48 | ||
One of the most important environmental problems faced by the world is management of wastes. Industrial processes generate a variety of molecules that may pollute air and waters due to negative impacts such as Toxicity, Carcinogenic and Mutagenic properties. The aim of the present paper is, it provides an outline of the Physico-Chemical characteristics, hazards and remediation measures for control of phenol toxicity. Phenol and its components are extremely toxic and can be easily be isolated from different industrial sewage such as oil refinery, petrochemical industry and mines, especially collier and chemical factories. Hence, the presence of these compounds in the environment could cause environmental pollution, especially in water resources. The purpose of the study is crucial to perpetuate the environment and individual. Various methods are recommended for removal of the phenol from wastewater include physico-chemical and biological like steam stripping, solvent extraction, adsorption, chemical oxidation and biodegradation methods are discussed. The work reported in this study is, the complete removal of the pollutants by the use of physical and chemical processes is not possible. Critical appraisal of the literature reveals that, biological treatment is economical, practical and the most promising and versatile approach as it leads to complete mineralization of phenol producing non toxic end products.
Organic pollutants comprise a potential group of chemicals which can be dreadfully hazardous to human health. Many of these are resistant to degradation. As they persist in the environment, they are capable of long range transportation, bioaccumulation in human and animal tissue and biomagnification in food chain (Indu Nair C., Jayachandran, K. and Shankar Shashidhar, 2008).
Now-a-days, there are increasingly stringent regulations requiring more and more treatment of industrial effluents to generate product waters which could be easily reused or disposed off the environment without any harmful effects. Therefore, different advanced processes were investigated as suitable precursors for the efficient treatment of industrial effluents containing phenol.
For removal of phenol, extensive research has been carried out to study the removal potential of each of the method. Many biological and nonbiological processes such as Polymerization, Electro Coagulation, Extraction, Photo decomposition, Advanced Oxidation Process and adsorption were employed by various researchers for phenol removal. This paper shows a promising research way for the development of efficient coupled processes for the treatment of wastewater containing toxic or biologically non-degradable compounds.
Man-made chemicals present in the nature at high concentrations polluting the environment or chemicals that are foreign to the biosphere are known as xenobiotic compounds. These compounds are not commonly produced by nature. Some microbes have been seen to be capable of breaking the xenobiotics to some extent. But, most of the xenobiotic compounds are non degradable in nature. Such compounds are known to be recalcitrant compounds in nature.
Release of chemical substances due to rapid industrial progress has now become a serious problem causing environmental pollution. Pollutants resembling structural features of xenobiotics mostly include organic sulfonic acids, halogenated aliphatic and polycyclic aromatic hydrocarbons, s-triazines, nitroaromatic compounds, azo compounds and synthetic polymers. Over the years, huge quantity of hazardous waste sites are being generated throughout the world due to the accumulation of xenobiotic compounds in soil and water. Polycyclic Aromatics, Nitroaromatic Compounds (NACs), and other Hydrocarbons (PAHs) constituting crude oil, are among the diverse group of xenobiotic chemicals responsible for immense environmental pollution.
Phenol and its derivatives are common water pollutants and include wide variety of organic chemicals (Santos V.L., Valter R. Linardi, 2004). These are aromatic molecules containing hydroxyl group attached to the benzene ring structure. Wastewater with high concentrations of phenol can be treated by physio-chemical or biological methods. Physico-chemical treatments using adsorption with bone char or zeolites, stripping with air or steam, wet air oxidation or biological treatments with mixed and pure cultures of microorganisms, activated sludges and anaerobic cultures (Nurdan Kas et.al, 2005).
Phenol is a waste product of industrial processes that is introduced into aquatic ecosystems, adversely affects the indigenous biota, including algae, protozoa, invertebrates, and vertebrates (Babich and Davis, 1981). The concentrations of these compounds ranges from one to several hundred mg/L (Moussavi et al. 2008). Industrial wastewaters associated with the manufacture of halogenated organics characteristically have concentrations as high as hundreds of mg/L (Annachatre and Gheewala 1996). Water pollution by organic and inorganic compounds is the great public concern (Pradeep et al. 2014 and 2015). But in real, they have great importance as they are toxic, recalcitrant and bioaccumulating in organisms (Annachatre and Gheewala 1996).
Figure 1 shows, the chemical structure of phenol. Phenol (C H OH) is the monohydroxy derivative of Benzene and is 6 5 a clear, colorless-to-white solid, hygroscopic in nature (Srihari.V and Ashutosh Das, 2014). It was first isolated from coal tar in 1834 and was named carbolic acid. It is also called as Benzenol, Hydroxybenzene, Monophenol, Oxybenzene, Phenyl Alcohol, Phenyl Hydrate, Phenyl Hydroxide, Phenylic Acid and Phenylic Alcohol. Phenol has a distinct odor that is sweet and tarry. The physical and chemical properties of phenol are listed in Table 1.
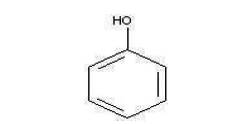
Figure 1. Structure of Phenol
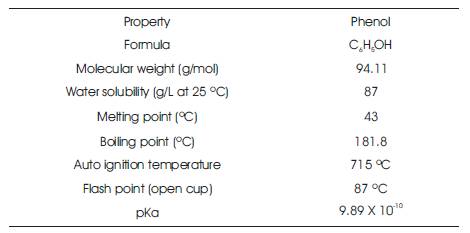
Table 1. Chemical and Physical Properties of Phenol (Kirk-Othmer, 1999)
The origin of phenol in the environment is from natural, man- made and endogenous sources.
Phenol is a constituent of coal tar, and is formed during decomposition of organic materials. Natural sources of phenol include forest fire, natural run off from urban area, where asphalt is used as the binding material and natural decay of lignocellulosic material.
Phenols are common components of industrial streams, from polymeric resin producing companies, coal gasification, oil refining, and coking plants. Process industries are major sources of phenolic discharges such as petroleum refineries, gas and coke industries and fibre glass units. Smelting and connected metallurgical operations, plastic industries, pharmaceutical and chemical industries, paint, pulp and paper mills (Kumaran & Paruchuri, 1997), vanish industries and textile units making use of organic dyes also contribute to phenolic liquid waste (Gurusamy Annaduraia et.al, 2002).
An important additional source of phenol may be the formation from various xenobiotics such as, benzene (Pekari et al. 1992) under the influence of light.
The main use of phenol is, it is as an intermediate in the production of phenolic resins. However, it is also used in the production of caprolactam, which is mainly used in the manufacture of nylon 6 and other synthetic fibers and bisphenol A, which is widely used to produce polycarbonate plastics, dyes, epoxy coatings and flame retardants.
The primary use of phenol is in the production of phenolic resins, which are used in the plywood, construction, automotive, and appliance industries. Phenol can have beneficial effects which include ointments, ear and nose drops, cold sore lotions, mouth washes, gargles, toothache drops, analgesic rubs, throat lozenges and antiseptic lotions. Phenol is the main ingredient in synthetic vanilla flavouring. Phenol is used in a wide variety of medical applications ranging from aspirin to throat lozenges and sprays. Phenol derivatives are used in laser and ink jet printers as a coating for the ink. Many pipes have phenolic or epoxy coatings to prevent corrosion. The other derivatives of Phenol (o-cresol, m-cresol, p-cresol and Pentachlorophenol) are also used as a slimicide, which is a chemical toxic to bacteria and fungi characteristic of aqueous slimes and as a wood preservative. The main anthropogenic sources of phenol in natural water include, coal tar and wastewater from manufacturing industries such as resins, plastics, fibres, adhesives, iron, steel, aluminum, leather, rubber, effluents from synthetic fuel manufacturing, paper and pulp mills and wood treatment facilities. Other releases of phenol result from pharmaceuticals, disinfectants. In 1996 the total release of 414.7 tonnes of phenolics were reported in Canada and its world production reached 7.8 million tonnes in 2001. Phenol ranks in the top 50 in production volumes for chemicals produced in the United States. Phenol has been widely produced mainly by two processes, oxidation of Cumene or toluene and by vapour phase hydrolysis of Chlorobenzene.
Disinfectants are antimicrobial agents that are applied to non-living objects to destroy microorganisms that are living on the objects. Disinfectants are different from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides, the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with the metabolism. Phenolic compounds used as antiseptics or disinfectants include, pure phenol and substitution products with halogens and alkyl groups. They act to denature and coagulate proteins and are general protoplasmic poisons.
Phenol (carbolic acid) is one of the oldest antiseptic agents. It is bacteriostatic at concentrations of 0.1%–1% and is bactericidal/ fungicidal at 1%–2%. A 5% solution kills anthrax spores in 48 hours. The bactericidal activity is enhanced by EDTA and warm temperatures; it is decreased by alkaline medium (through ionization), lipids, soaps, and cold temperatures. Concentrations >0.5% exert a local anesthetic effect, whereas a 5% solution is strongly irritating and corrosive to tissues.
Phenol has occupied a prominent place in the field of hospital disinfection; since, its initial use as a germicide by Lister in his pioneering work on antiseptic surgery. In the past 30 years, however, work has concentrated on the numerous phenol derivatives or phenolics and their antimicrobial properties. Phenol derivatives originate when a functional group (e.g., alkyl, phenyl, benzyl, halogen) replaces one of the hydrogen atoms on the aromatic ring. Two phenol derivatives commonly found as constituents of hospital disinfectants are orthophenylphenol and ortho-benzyl-para-chlorophenol. The antimicrobial properties of these compounds and many other phenol derivatives are much improved over those of the parent chemical. Phenolics are absorbed by porous materials, and the residual disinfectant can irritate tissue. In 1970, depigmentation of the skin was reported to be caused by phenolic germicidal detergents containing para-tertiary butyl phenol and para-tertiary amylphenol. Many phenolic germicides are EPA-registered as disinfectants for use on environmental surfaces (e.g., bedside tables, bedrails, and laboratory surfaces) and noncritical medical devices. These are not FDA-cleared as high-level disinfectants for use with semicritical items but could be used to preclean or decontaminate critical and semicritical devices before terminal sterilization or high-level disinfection.
There is a long history of human exposure to phenol. Acute exposure of phenol causes central nervous system disorders. It leads to collapse and coma. Muscular convulsions are also happened. A reduction in body temperature is resulted which is known as hypothermia. Mucus membrane is highly sensitive to the action of phenol. Muscle weakness and tremors are also observed. Acute exposure of phenol can result in myocardial depression.
Phenol causes a burning effect on skin. Whitening and erosion of the skin may also result due to phenol exposure. Phenol has an anaesthetic effect and causes gangrene. Renal damage and salivation may be induced by continuous exposure to phenol. Exposure to phenol may result in irritation of the eye, conjunctional swelling, corneal whitening and finally blindness. Other effects include frothing from nose and mouth followed by headache. Chronic exposure may result in anorexia, dermal rash, dysphasia, gastrointestinal disturbance, vomiting, weakness, weightlessness, muscle pain, hepatic tenderness and nervous disorder. It is also suspected that, exposure to phenol may cause paralysis, cancer and gene to fibre striation.
Methemoglobinemia and haemolytic anaemia, are as liver damage, have also been reported following human exposure to phenol. The odour threshold has been 3 reported to range from 0.021 to 20 mg/m in air, while the threshold for odour in water has been reported to be 7.9 ppm. A taste threshold value of 0.3 ppm water has been suggested. Phenol and its derivatives are toxic and classified as hazardous materials (Zumriye and Gultac, 1999) and it is toxic to both aquatic and terrestrial life ( (Kanekar P. P., Sarnaik S. S. and Kelkar A.S.,1999). Studies in humans and animals indicate that, most of the phenol that enters into the body through skin contact, breathing contaminated air, eating food or drinking water, or using products containing phenol, leaves the body in the urine within 24 hours. The normal range of phenol in the urine of unexposed individuals is 0.5-80 milligrams of phenol per litre of urine, and also the toxic levels are different for different organisms as shown in Table 2.
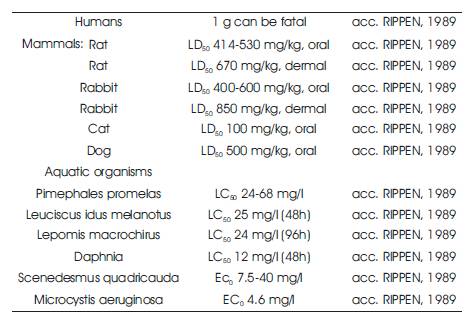
Table 2. Different Toxic Levels of Different Organisms
Pollution of environment is one of the major challenges of today's civilization. Phenol is a naturally occurring and manufactured chemical that is widely distributed in the environment as pollutants due to their common presence in the effluents of many industrial processes (Butani Naresh, Parekh Honey and Saliya Vaishali, 2012). They will do harm to the environment and have been listed in the 129 priority pollutants. It is an antiseptic and is used in surgery, which indicates that they are also toxic to many microorganisms (EPA, 1979). The most likely route of exposure to phenol is through dermal contact either in the work environment or at home using ointments and other household products containing phenol. It is released from a number of man-made sources; but, the diffuse emissions by automobile exhaust, human and animal metabolism and different combustion processes are turn out to be higher than the industrial emissions (ECB, 2006). Phenol is non-persistent in the environment and the major part of phenol in the atmosphere is degraded by photochemical reactions. A minor part will be removed by rain. Phenol in water and soil is degraded by abiotic reactions and microbial activity.
It is a product of combustion of coal, wood and municipal solid waste; therefore, residents near coal and petroleum fuelled facilities as well as residents near municipal waste incinerators may have increased exposure to phenol. They induce genotoxic, carcinogenic, immunogenic, haematological and physiological effects and have a high bioaccumulation rate along the food chain due to its lipophilicity (Bin Cao & Karthiga Nagarajan & Kai-Chee Loh, 2009).
As water is a precious commodity, according to the standard set by United State Environmental Protection Agency (USEPA), surface water must contain less than 1 microgram/litre phenol. In this regard, industrial effluents containing phenols require proper treatment prior to discharge into the environment. There have been many studies on the toxicity of phenol to organisms in the environment, covering short-term and long-term toxicity to fish/vertebrates, invertebrates, short term toxicity to algae and toxicity to microorganisms.
In recent years, a great deal of research work has been directed toward the development processes in which, enzymes are used to remove phenolic contaminants (Ghioureliotis and Nicell, 1999). Thus phenol pollution represents a threat against natural environment and also human health. The concentrations of phenol in different indusrial effluents are shown in Table 3.
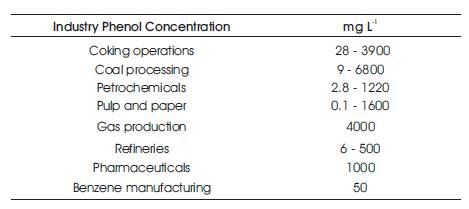
Table 3. Phenol Concentrations in Industrial Effluents (Busca et al. 2008)
Phenols in Industrial effluent have teratogeny, carcinogenic mutagenic effects. They are toxic and difficult to bio-degrade. Many of the industrial effluents containing priority organic pollutants exhibit high salt concentrations and/or the extremes of pH, one or both of which prevents microbial growth or make it very difficult to sustain (Ruey-Shin Juang et.al., 2009). The non- treated industrial effluents with phenols have threatened the living environment of humans and animals. Nowadays, the treatment of effluents with phenols have sparkled the concern of researchers. A variety of treatment methods, such as incineration, adsorption, wet oxidation, biological treatments and chemical oxidation have been used for removal of phenols from aqueous solutions
Incineration of organic waste is particularly useful for treating small quantities of wastes with high pollutant concentrations. The incinerators normally used could be horizontal, vertical or fluidized bed vessels. Incineration of organic waste presents the disadvantage of high investment costs for equipment and high operating costs for energy because of additional fuel requirements. Another drawback of this treatment is the production of CO2 and NOX resulting from the oxidation of organic compounds at elevated temperatures.
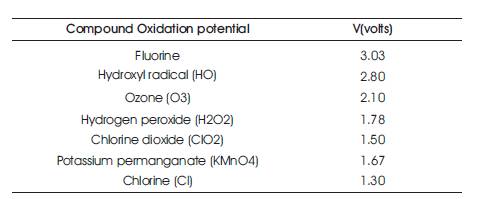
Table 4. Standard Oxidation Potential (against the Standard Hydrogen Electrode Commonly used Chemical Oxidants (Legrini et al. 1993)
Air, chlorine, ozone, and other chemical oxidizing agents are used to destroy phenol, which is first converted to hydroquinone and then to quinone. Additional oxidation destroys the aromatic ring, forming organic acids and eventually carbon dioxide and water. Air is an inexpensive oxidizing agent; but, reactions are slow. Phenol can be completely decomposed by chlorination at pH 7.7, provided that, the stoichiometric amount of chlorine is added. This is accomplished in water treatment plants by super chlorination. The major portion of the chlorine applied consumes other organic compounds and destroys ammonia. Approximately 42 parts of chlorine per part of phenol are required. Ozonation effectively oxidizes phenol. However, the initial cost of producing ozone is high. Ammonia does not interfere in ozonation, and approximately 5.8 parts of ozone are required per part of phenol.
Chemical oxidation can be divided into either classical chemical treatments or Advanced Oxidation Processes (AOPs). Classical chemical oxidation is the direct addition of the oxidant to the wastewater containing pollutants. The following are the most common chemical oxidants used for this purpose. The standard oxidation potential of commonly used chemical oxidants are shown in Table
Coagulation is the formation of small flocs from dispersed colloids using coagulating agents. The major disadvantage of coagulation / flocculation processes is the production of sludge and subsequent separation and removal of it.
Flocculation is the agglomeration of small flocs into larger settleable particles using flocculating agents.
Activated carbon in the powdered and granular forms is used to remove phenolic tastes and odors from drinking water supplies. In wastewater treatment applications, where phenol content is considerably greater than in potable water applications and the flow is continuous, granular carbon systems are more economical.
Depending on the concentration of phenol and other organic compounds in the wastewater, activated carbon will adsorb from 10 to 25 gm of phenol per 1000 gm of carbon. This capacity can be determined from isotherm and column test data. In general, phenol adsorption improves as the pH decreases. Adsorption at high pH is poor, since phenolate salt forms and is difficult to adsorb. This is an advantage in applications, where phenol recovery is worthwhile.
Enzymes are proteins and are found in all types of cells. Enzymes are biological catalysts; the reactions speeded up one million times faster than the rate in the absence of enzymes. Peroxidases are enzymes that catalyze the oxidation of organic and inorganic compounds. The enzyme catalyzed reaction for phenolic wastes may be as follows:
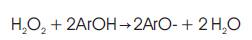
where ArOH is the Phenolic waste and ArO is a phenoxy radical, which is highly reactive and develops insoluble polymer, that can be removed by filtration.
The process of treating, impregnating or combining with ozone. The main disadvantage of this process lies in the high cost of reagents.
In Kopper's method of steam stripping, dephenolation takes place at 1000° C and stripped phenol absorbed by 15% sodium hydroxide solution. It is estimated that 1000 m3 of wastewater would require 200 tonnes of steam and 2 tonnes of sodium hydroxide.
It involves the oxidation of organic matter in water in the presence of oxygen (or air). The reaction takes place above the water boiling point (100°c) but below the critical point (374°C).
Phenols are weak acids and can be extracted with various basic and solvating reagents or removed from aqueous solution by activated carbon or anion-exchange resins. The drawback of the former liquid–liquid extraction technique is the possibility of the diluents and extractants which constitute the organic solvents into which the pollutants are extracted being the source of an induced pollution. Thus, new separation methods, including the techniques of micellar-enhanced ultrafiltration based on the coupling of micellar extraction and ultrafiltration, separation through membranes by pervaporation, novel solvent extraction, electrocoagulation have been proposed and found to be a promising processes for the elimination of pollutants, including phenols, from aqueous solutions.
A paraffinic- based solvent (Iso-Kerosene) was used for the removal of phenolic contaminant from water and wastewaters. It is a novel industrial solvent. For wastewaters containing high phenol concentrations, solvent extraction reduces the phenol to acceptable levels. In solvent extraction, two immiscible or partially soluble liquids are brought into contact for transfer of one or more components. The extraction of phenols by using solvents is the best method when concentration of phenol is high in the solution. The most popular solvents are Benzene, light tar oil, hydrogenated tar oils ester groups, phenosolvan (higher Partition coefficient than benzene), etc. The extracted phenol is then washed out with caustic to form the sodium salt and the benzene is reused. In the petroleum industry, light catalytic cracking oils are used as extractors, and in the coking industry, coke oven light oils are used as extractors. Process efficiency depends on solvent choice and system design. The extraction methods are uneconomical if the flow rates are at least 200m3 /day and 350 m3 /day in benzene– NaOH method and phenosolvan method respectively for a minimum phenol concentration of 2000 ppm.
In recent years, Cyclodextrins (CyDs) have attracted considerable attention for the removal of organic pollutants from aqueous streams; because, the cavity in their structure, is hydrophobic in nature and is capable of binding aromatic and other small hydrophobic organic molecules, and therefore provides ideal binding sites. Because of their solubility in water, CyDs cannot be used directly for separation, and hence it seemed plausible that insoluble CyD polymers would be good candidates for a recyclable adsorbent, which selectively binds to phenols in water. Removal of phenolic compounds from a raw industrial wastewater from phenolic resin processing, of which the components are phenol, m- and p-cresols, and xylenols, was carried out by using crosslinked cyclodextrin particles as a sorbent. This novel CyD polymer remove phenols from raw industrial wastewater discarded from phenolic resin processing that is more effective than those available at present.
Biological treatment of wastewater containing organic pollutants is the natural and economic alternative compared to other treatment options. The cost of biodegradation of organic contaminants is reported to be 5 to 20 times less than chemical treatments such as ozonation and hydrogen peroxide (Mantzavinos et al., 1999).
Bioremediation involves the use of specific microorganisms to degrade the organic pollutants in the wastewater and tailings. The microorganisms need carbon (organic contaminants) as a source of energy and inorganic salts or nutrients to reproduce and carry out their metabolic activities. The organic contaminants in wastewater serve as carbon and energy sources for the microorganisms. The main nutrients used to maintain the microbial activity are Nitrogen (N), Sulphur (S), Potassium (K), Magnesium (Mg) and Calcium (Ca). Besides the organic contaminant and nutrients, biodegradation of an organic pollutant is dependent on several other factors such as pH, temperature, availability of oxygen in case of aerobic processes and concentrations and chemical structure of the target pollutant. The microorganisms may completely destroy or convert these contaminants to harmless or simple inorganic compounds such as water and CO2.
The clean-up of the Alaskan shoreline of Prince Williams sound after the oil spill of the Exxon Valdez in 1998 is an example of a large scale application of bioremediation that got much public attention (Boopathy, 2000).
Bioremediation can be classified as in-situ or ex-situ. The ex-situ approach involves the physical removal of the contaminated waste from the original location and conducting the treatment in a bioreactor. In-situ involves treatment of the contaminated waste in place. Although the in-situ approach is less expensive, the ex-situ approach may provide higher efficiencies as a result of carefully controlled conditions in the bioreactor. The exsitu approach has been found to be more predictable and to be controlled easier than in-situ bioremediation (Carberry and Wik, 2001).
Numerous studies have been reported in the literature on the biodegradation of phenol using different microorganisms. Degradation of phenol occurs as a result of the activity of a large number of microorganisms including bacteria, fungi and actinomycetes, Bacterial species include Bacillus sp, Pseudomonas sp, Acinetobacter sp, Achromobacter sp, etc.
Fusarium sp, Phanerocheate chrysosporium, Corious versicolor, Ralstonia sp, Streptomyces sp etc., are also proved to be efficient fungal groups in phenol biodegradation. Many studies on biodegradation of phenol comes from the bacteria. The genus Pseudomonas is widely applied for the degradation of phenolic compounds. These bacteria are known for their immense ability to grow on various organic compounds. Phenol biodegradation studies with the bacterial species have resulted in bringing out the possible mechanism and also the enzyme involved in the process.
Recent advances in membrane based technology has brought better choice to chemical processing industries for the purification, concentration, and fractionation of a variety of fluid mixtures. Pervaporation is an energy saving membrane process in which, a liquid mixture is to be separated (feed) and is placed in contact with one side of a membrane and the permeated product (permeate) is removed as a low-pressure vapor from the other side. This technique would allow removal of a considerable part of the organic pollutants like phenol, whereas adsorption, the classical separation technique, in which organic pollutants are removed from the wastewater by adsorption onto the surface of solid particles where it is accumulated for further extraction or destruction. It would lower the phenol concentration of the treated effluent to the level acceptable by wastewater treatment plant. So, recent advances in the application of this hybrid process to the wastewater efficiently remove the phenol using cumine oxidation process (Wojciech Kujawski et al, 2004).
Electro-coagulation uses an electrochemical cell to treat polluted water. A sacrificial metal anode (usually aluminum but sometimes iron) is used to dose polluted water with a coagulant agent. Compared with traditional chemical coagulation, electrocoagulation has, in theory, the advantage of removing the smallest colloidal particles; the smallest charged particles have a greater probability of being coagulated, because of the electric field that sets them in motion. It has also the advantage of producing a relatively low amount of sludge. Electrocoagulation is an ideal technology to upgrade water quality (Ahmed AMohammed, 2007).
Electrodes for wastewater treatment are still the high energy consumption associated with these processes. Even considering the best operational conditions at which, the lowest energy consumptions were obtained, these values are still very high and need to be reduced in order to make the electrochemical technology suitable to be used in a large-scale wastewater treatment plant. In one approach to reduce the energy consumption, electrochemical technology could be used only to oxidize the non biodegradable aromatics, so that the resulting compounds could be sent to biological treatment (Britto-Costa P.H. etal., 2012). In addition, an electromagnet will be applied to the clean-up of contaminated water and soil with various pollutants as well as phenol at pilot scale in an ongoing study (Sung Ho Yeoma et al, 2010).
As a solution, hybrid processes have been developed that combine the pressure driven membrane and adsorption processes for the separation and/or removal of phenols. Because of their low molecular weights, simple ultrafiltration is ineffective for retaining these compounds. Micellar Enhanced Ultrafiltration (MEUF) could be considered for the retention of these pollutants. In such a process, a surfactant is added into the aqueous stream containing organic matters. When the surfactant concentration rises above the Critical Micellar Concentration (CMC), surfactant monomers assemble and aggregate to form micelles. These macromolecular structures can solubilize organic matters into their hydrophobic core or adsorb on its surface. The aqueous stream is then filtered by an appropriate ultrafiltration membrane with pore sizes, which is smaller than the micelle size. The micelles along with the solubilized organic matters are then rejected into the retentate stream. The MEUF process has been recently reported to separate different organic compounds: a-Phenylglycine, lactic acid, tannic acid, aromatic alcohols, methylene blue , and phenols (Abdelilah El-Abbassi et.al., 2014).
It consists of hybrid process which would involve both conventional separation methods (distillation, adsorption) and membrane separation techniques (pervaporation and/or membrane-based solvent extraction) for the treatment of effluents from the cumene oxidation process. This process consists in oxidation of isopropyl benzene (cumene) with air, followed by cleavage of the formed cumene hydroperoxide in the presence of an acid catalyst (Wojciech Kujawski et.al., 2014). However, the cumene oxidation process is also a source of wastewater. These membrane techniques should allow removal of a considerable part of the organic pollutants, whereas adsorption should lower the phenol concentration of the treated effluent to the acceptable level by wastewater treatment plants.
The AOPs use ozone, UV, ozone in combination with UV (O3/UV), ozone plus hydrogen peroxide (O3/H2O2), hydrogen peroxide and ultraviolet light (UV/H O ). The main problem of AOPs lies in the high cost of reagents such as ozone, hydrogen peroxide or energy light sources like ultraviolet lights. (Sunil J. Kulkarni, and Jayant P. Kaware, 2013). And also different advanced oxidation processes were investigated as suitable precursors for the biological treatment of industrial effluents containing phenol (Rubalcaba. A et al, 2007)
The Combined UV/O3 process is an advanced oxidation treatment for effective oxidation and removal of organic contaminants from wastewater. This process has been used at large scale for the destruction of toxic and refractory contaminants and also for the disinfection of drinking water by destroying bacteria and viruses.
Photocatalytic oxidation has been an efficient and promising alternative for the destruction of pollutants from wastewater.
In 1894, Fenton found that a combination of ferrous salts and hydrogen peroxide was able to rapidly oxidize maleic acid. Since then, the addition of ferrous salts to hydrogen peroxide has been used to promote the oxidation of a variety of organic compounds.
The wastewater is enriched with varied pollutants and harmful to both human being and the aquatic flora and fauna and its successive accumulation in the soil has adverse effect on soil productivity. Phenol is a major pollutants being discharged from the effluents of various sources. They mix in the water bodies and make them unusable. Phenol is non-persistent in the environment and the major part of phenol in the atmosphere is degraded by photochemical reactions. A minor part will be removed by rain. Phenol in water and soil is degraded by abiotic reactions and microbial activity. Several physical chemical and biological methods for removal or treatment of phenol are in use. In the present study, focus is given on new separation methods, including the techniques of micellar-enhanced ultrafiltration based on the coupling of micellar extraction and ultrafiltration, separation through membranes by pervaporation, novel solvent extraction, electrocoagulation have been proposed and found to be promising processes for the elimination of pollutants, including phenols, from aqueous solutions. The paper describes the results of an investigation aimed at evaluating suitability. The environment, as a consequence of industrial and agricultural revolutions, tends to harden with potentially carcinogenic and mutagenic halogen-substituted aromatic compounds. Phenol and its higher molecular homologues are dangerous environmental pollutants. Due to their toxic character, these molecules tend to accumulate in water and soil after being discharged without an adequate treatment. Physical and chemical methods have been designed to remove phenol from effluents but many of these methods are commercially impractical either because of their high operating costs or or of the difficulty encountered in treating the solid wastes generated.