 |
i-manager's Journal on Future Engineering and Technology |
View PDF |
|||
| Volume :11 | No :2 | Issue :-2016 | Pages :1-9 | ||
The authors report a novel detection technique for two types of common heavy metal contaminants in water, copper sulphate and ferric chloride, by analyzing the transmittance measurement of a supercontinuum laser source on the samples. In the past, the detection of heavy metal contaminants in water was done conventionally by using atomic spectroscopy method. This method requires a series of steps such as atomization of water sample by burning the sample with flame and then shines a specific wavelength of light on that cloud of atoms in order to determine the presence of that particular heavy metal contaminant. The authors aim to demonstrate a more convenient method, which is by shining a broadband supercontinuum light source directly on the water sample and performing transmittance measurement. Instead of shining a light source with certain wavelength, supercontinuum laser source allows emission of light ranging from 600 nm to 1600 nm wavelength simultaneously. By measuring the amount of light transmitted through the water sample, the concentration of heavy metal contaminants can be determined. The laser source, which comprises of a Master Oscillator Power Amplifier (MOPA) paired with a 15 meters long Photonic Crystal Fiber (PCF) has the ability of producing broad bandwidth light source with an average power of 1W. The experimental results were further analyzed using Analysis Of Variance (ANOVA) in which, the authors have identified that wavelength, concentration of heavy metal contaminants and the types of contaminants that will affect the transmittance of the supercontinuum laser source.
Critical issues regarding water pollution have become increasingly significant in recent years due to the presence of heavy metals that poses risks to human health and also to the environment. Industrial activities such as mining and construction in the vicinity of water sources (river or lake waters) caused the increase of heavy metals concentration in these river or lake waters due to indiscriminate release of industrial waste into the waters [1]. Typical heavy metals which is present in water such as copper, iron, cadmium, zinc and chromium will cause harmful effects such as diarrhea, vomiting or even cancer and birth defects if exposed excessively to the human body for a long term. Therefore, methods for monitoring the concentration of heavy metals in water are required in order to make sure that, the concentration of heavy metals in water is within the limit so that, the water is safe for human consumption.
Conventional methods for detecting heavy metals in water are Atomic Absorption Spectroscopy (AAS), Inductively Coupled Plasma – Atomic Emission Spectrometry (ICP-AES) and Laser Induced Breakdown Spectroscopy (LIBS). Atomic absorption is based on the excitation of atom from ground state to excited state upon absorbing energy from a specific wavelength of light. Performing AAS requires a primary light source such as, hollow cathode lamp or electrodeless discharge lamp, an atom source that produces free atoms from the water sample, a monochromator to isolate the specific wavelength of light to be measured, and a detector to measure the light accurately. As the number of atoms of the selected element in the light path increases, the amount of energy absorbed at that particular wavelength increases as well. Therefore, by measuring the amount of light being absorbed, the concentration of contaminants present in an unknown solution can be determined [2] . Mehrorang Ghaedi et al. have proposed a method of determining heavy metal ions after solid phase extraction by using flame AAS [3].
Conversely, ICP- AES is an optical emission spectrophotometric technique. It measures the emission of excited electrons at a given wavelength, as they return to ground state. ICP-AES involves injecting an Argon (Ar) gas along with the sample solution to be tested into the Ar plasma maintained by a Radio Frequency (RF). Due to the high temperature of the Ar plasma (around 8,000 K to 10,000 K), the electrons are being excited from ground state. After the electrons cool down, they emit energy in the form of light at specific wavelength. The emission of light from the plasma is collected and focused into a spectrometer that separates the emitted light into its discrete component wavelengths. Charged Coupled Detectors (CCD) are used to quantify the amount of light at a given wavelength [4]. Each element has its own emission wavelength. Therefore, the concentration of a particular element can be determined by measuring the intensity of energy emission at particular wavelength. Hassan Karami et al. proposed a method by using flow injection analysis system for on-line preconcentration and simultaneous determination of heavy metal ions in aqueous samples by ICP-AES with a CCD [5].
On the other hand, LIBS is another technique which uses emission spectroscopy. The sample to be tested is heated up and turned into a gaseous plasma state when a high intensity laser beam is focused onto it. The light emitted by the gas plasma which is in the form of atoms is being collected and analyzed by a spectrophotometer. The intensity of the light given off at different wavelengths allows the user to determine the concentration of the sample [6]. Senesi et al. have demonstrated on determining the concentration of heavy metal in soils by LIBS [7].
In this work, the authors aim to trace the amount of heavy metals in water by measuring the transmittance upon illuminating a Supercontinuum (SC) light source onto the water sample. This proposed method is expected to be easier to conduct than the conventional method, as there is no atomization of water samples required. The SC generation involves generation of a broadband laser source, which occurs when narrow-band incident pulses are typically in picoseconds range undergo extreme nonlinear spectral broadening, emitting light simultaneously in the UltraViolet (UV), visible and Infrared (IR) ranges [8]. SC has been used in several applications such as optical frequency combs, nonlinear microscopy and wideband spectroscopy [9]. The SC beam is focused on to the sample solution containing heavy metal contaminants and the intensity is recorded and compared against the intensity spectrum of sample solution without contaminants (pure water). This method is also known as the light attenuation measurement. When the light beam passes through a solution, some of the light may be absorbed with the remainder transmitted through the sample. A.F. Omar et al. have demonstrated a similar method of measuring the transmittance of different concentration of clay through optical fiber sensor with Light Emitting Diode (LED) as the light source [10] . According to Beer-Lambert law, the absorbance of a solution is directly proportional to the concentration of the absorbing species in the solution and also the path length [11]. In other words, by fixing the path length, the concentration of the absorber in a solution can be determined. This is because of the concentration of contaminants in water increases, the presence of particles from the contaminants will increase as well. Subsequently, the particle will absorb a portion of light, preventing the light from transmitting through the water, and causing the intensity of transmitted light to decrease. Ultimately, the difference in the intensity of transmitted light for different sample solutions can be related to the concentration of the contaminants.
A SC laser system consists of a Master Oscillator Power Amplifier (MOPA) with Photonic Crystal Fiber (PCF) is shown in Figure 1. MOPA refers to configuration consisting of a laser seed and optical amplifiers to boost the output optical power [12]. By connecting this MOPA system with PCF, the supercontinuum light source can be generated easily due to the high optical peak power provided from the MOPA laser system [8]. In this MOPA system, it comprises a seed laser stage (initial) followed by two subsequent preamplifier stages and a power amplifier stage. The seed generates sub nanosecond optical pulses with 150ps pulse width at 1 MHz repetition frequency. Given that, the average power is around -15 dBm, cascaded fiber amplifiers are required to amplify the power signal and increase the gain significantly to around 30 dBm. The output of MOPA system was attached to a 15 meters long PCF. Initially, the narrow spectrum at 1060 nm wavelength was having an average power of 0 mW. With the aid of PCF with high nonlinearity characteristics, the spectrum starts to broaden as the power inreases, up to 1W of optical power can be achieved in this laser system. The formation of broadband continuum across wavelength 600nm to 1600nm is shown in Figure 2. The Y-Axis represents 10dBm per division The authors used the PCF which is made specially for this experiment. It has a core diameter of 4.8 μm and cladding diameter of 125 μm with Numerical Aperture (NA) of 0.2. Besides, it operates at single mode with zero dispersion wavelength of 1040 nm, which allows the spanning of spectrum of the MOPA system that operates at 1060 nm. The cross section of PCF is shown in Figure 3, which is used to generate supercontinuum Laser Source.
Figure 2. Formation of Supercontinuum Broadband Spectrum At Different Output Power across the Wavelength 600 nm to 1600 nm obtained from Optical Spectrum Analyzer (OSA).
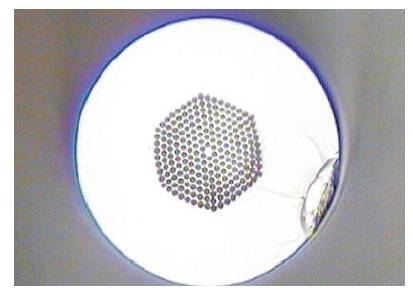
Figure 3. Cross Section of a Photonic Crystal Fiber (PCF)
The experiment setup is shown in Figure 4 with SC laser serves as the light source. The average power of SC source is fixed at 1W with repetition rate of 1 MHz. The output of SC is fed into a specially designed glass with 10 cm optical path length. This glass medium allows continuous flowing of the sample solution as shown in Figure 5. The focusing lens is located at another end of the glass medium, which is used to couple all the transmitted collimating SC rays onto a multimode fiber, which is fixed on a translational stage. By adjusting the translational stage, a maximum coupling of the transmitted rays in the multimode fiber is achieved. The other end of the multimode fiber is connected to an Optical Spectrum Analyzer (OSA) that measures the transmitted supercontinuum light.
Two different types of sample solutions were used in this experiment. They are copper sulphate solution and ferric chloride solution. These two solutions are not typical contaminants discharged from the industrial waste, but they are very similar to the heavy metal industrial wastes disposed by the industry into the water as they are made up from metal ions as well, such as Copper (Cu) and Iron (Fe). According to Material Safety Data Sheet (MSDS), copper sulphate solution is harmful when consumed. It can cause liver or kidney damage if swallowed. On the other hand, ferric chloride solution is labelled as corrosive to eyes and skins. It is an acidic solution with a pH value of 2 [13 - 14].
This experimental setup is specially designed for on-site, remote, self-monitoring of river or lake water. This design allows the water sample to flow through the glass medium at all times and therefore a real time result can be obtained. The glass medium used in this experiment was fabricated specially for this experiment. According to Figure 5, there were two silica glasses attached in the glass medium. These two glasses ensure that, the water samples are flow in the designated direction. The reason for using silica glasses is, because, they are transparent and therefore SC light can be transmitted through the silica glasses and the water sample. Water sample flows into the glass through the opening at the top and leaves the medium via the opening at the bottom. The two glasses are used to block the water from flowing into wrong direction
Figure 6 shows the spectra measured using an OSA. The black bold line represents the emission wavelength from the SC light with wavelengths ranging between 800 nm and 1000 nm, whilst the dotted red line represents the transmitted spectrum measured at the output of the water flowing glass medium. In this experiment, the water samples that the authors used were typical filtered drinking water obtained from the standard utility water pipe. The authors observed that, the relative power intensity decreases in the presence of water. This is due to the absorption effect of the water molecules, reducing the amount of light passing through the water. The absorption of water molecules is different across the wavelength [15]. From Figure 6, it is found that, the absorption of water at the region of 950 nm is stronger as compared to the region at 900 nm, with approximately 13 dB differences between both optical wavelengths.
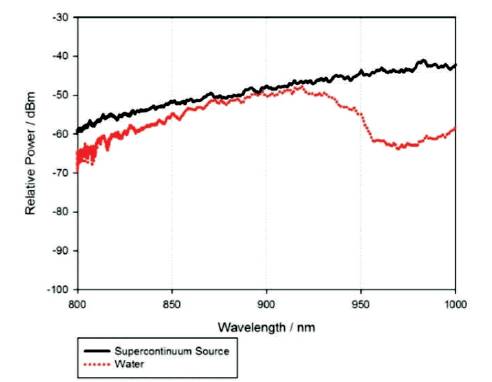
Figure 6. Optical Spectrum of Supercontinuum and Water
Next, the transmittance of the water sample is calculated based on equation (1),
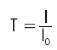
where I is the intensity of light coming out from the water sample, I0 is the intensity of incident light (SC source) and T is the transmittance ranging from 0 to 1.
Cháves et al. have demonstrated the transmittance of aqueous solution with heavy metals in the UV wavelength region, which is from 240 to 340 nm [16]. Here, the authors demonstrate a different approach by measuring the transmittance of water samples filled with heavy metals in visible-near-infrared region (800 to 900nm). Figure 7 and Figure 8 show the transmission spectra of pure water and water mixed with 60ppm and 120ppm of copper sulphate solution and ferric chloride solution respectively. As the concentration of contaminants increases, the absorption rate increases, as well as, due to the increment of particles in the water sample, the transmittance decreases. By judging both spectra, it is found that with the same concentration of contaminants (copper sulphate and ferric chloride) added into the water sample, the transmittance of water sample containing copper sulphate is lower compared to ferric chloride. This is because copper sulphate solution is capable of absorbing more light than ferric chloride solution due to the higher absorbance of copper ions than ferric ions across wavelength 800 nm to 900 nm [17]. In this experiment, the authors measured the water samples with concentrations from 20 ppm to 220 ppm with the resolution of 20 ppm per increment for copper sulphate. On the other hand, due to lower absorbance of ferric chloride compared to copper sulphate, the range of measurement is 40 ppm to 720 ppm concentration for ferric chloride with 40 ppm interval.
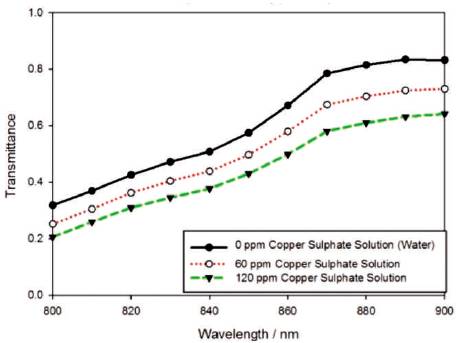
Figure 7. Transmittance Spectra of Different Concentration of Copper Sulphate Solution in Water
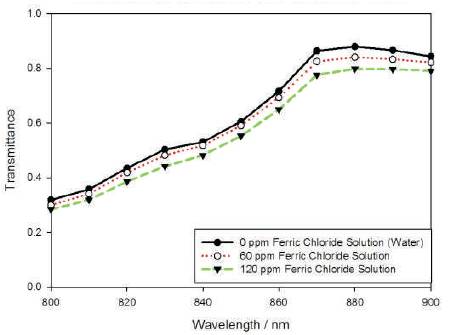
Figure 8. Transmittance Spectra of Different Concentration of Ferric Chloride Solution in Water
In order to determine the significant factors as well as the quantitative information of each factor in this experiment, a two level full factorial design with four factors (24) is carried out by using a Design Expert software. The four factors that are taken into the consideration are the wavelength of the SC source (800 nm to 900 nm), the concentration of the heavy metal contaminants (0 ppm to 120 ppm), types of contaminants (Copper Sulphate and Ferric Chloride) and input power (0.6 W to 1.0 W), whilst the response of the design was transmittance. The arrangement of each factor into its level is shown in Table 1.
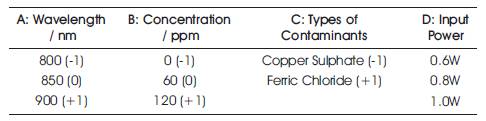
Table 1. The level of factors chosen for two level factorial design
In order to verify the significance of the factors chosen, a Pareto Chart is drawn as shown in Figure 9. Pareto chart is used to filter out the insignificant factors from the list by drawing two limit lines namely, the Bonferroni limit line and standard t limit line. Any value which is above the Bonferroni limit is considered as a certainly significant coefficient, whereas the value which falls between Bonferroni limit and standard t limit is categorized as likely significant. On the other hand, the values below the two limit lines are statistically insignificant and therefore, will be removed from the analysis [18-19].
The Pareto Chart indicates that the most significant factor is Wavelength (A), followed by Types of Contaminants (C) and then finally, Concentration (B). Wavelength is significant because at different wavelength, the absorption of heavy metal contaminants varies as well and therefore, affects the transmittance. Besides, different types of contaminants will also affect the transmittance. By comparing at the spectra from Figure 7 and Figure 8, the authors state that, copper sulphate solution has more impact to the transmittance compared to ferric chloride solution given that their concentrations are the same. Moreover, concentration of contaminants is also considered as a significant factor. The concentration of heavy metals in the water is inversely proportional with transmittance. As the concentration increases, more absorption occurs and therefore, lesser light is transmitted through the solution. Consequently, the transmittance decreases. On the other hand, input power factor (D) does not play an important role in this experiment as the transmittance is measured in term of ratio. Regardless the input power, the intensity of light coming out from the sample solution relative to the incident light will remain the same.
The factors that are labelled significant (A, B, C) according to Pareto Chart are chosen as the model for ANOVA analysis in order to determine the quantitative information of each factor as shown in Table 2. ANOVA is used to subdivide the total variation into variation due to main factors, variation due to interacting factors and variation due to error. The F-test from ANOVA can be used to help in screening many factors to discover the vital few and how they interact [20]. Based on Table 2, the model has F Value of 245.69, which implies that, the model is significant. There is only a 0.01% chance that a “Model F value” this large could occur due to noise. Moreover, when the p value is less than 0.05, it indicates that the particular model terms are statistically significant. In this case, the factors Wavelength (A), Concentration (B) and Types of Contaminants (C) are verified as significant model terms due to their p-values of less than 0.05 (A = <0.0001, B = 0.0002 and C = <0.0001 respectively). On the other hand, the model is also verified by the determination coefficient (R2), with the value of 0.9710 and it signifies that only 2.9% of the total variation is not explained by the model. The value of adjusted determination coefficient (adjusted R2) is 0.9671 which is also high, in order to show the high significance of the model [21-22].
According to Chavse et.al (2011), the best wavelength in determining heavy metal contaminants lies in the UV region (200 nm to 400 nm). However, due to the limitation of the measuring equipment, the lowest wavelength of spectrum, the authors could obtain was 600 nm only. Therefore, it is recommended to perform measurement at the UV region in order to obtain a more accurate result.
A broad Supercontinuum (SC) fiber laser source was used to investigate the optical transmittance of pure water and the water mixed with heavy metal solution. The water shows different optical absorption across the wavelength range from 800 to 900 nm and this signifies the difference in optical transmittance as well. As the heavy metal contaminants are introduced, the optical transmittance falls due to the more contaminant atoms absorbing the optical signal in the water sample, reducing the optical signal from passing through. A two level factorial design is conducted and it is found that, the wavelength, types of contaminants and concentration of contaminants are the significant factors, while the input power does not play an important role in this experiment. This is verified through this experiment. This experiment could be extended by developing a system, which is able to monitor the lake water as well as detecting heavy metal contaminants in river or lake water in real time.