Computerized detection of blood vessel structures is becoming one of the most interesting parts in the field of diagnosis of the vascular diseases. The objective of this paper is to introduce a novel filter, based on a new kernel function with Cauchy distribution to improve the accuracy of the automated retinal vessel detection. Moreover, for a good segmentation performance, the proposed model has the benefit of using distinct types of region information. The aim of the proposed model is to increase the accuracy of an image.
The vascular system is the body's network of blood vessels. It consists of the arteries, veins and capillaries that carry blood to and from the heart. Vascular diseases are the challenging issues in the present society. The proposed model is recommended for the detection and analysis of the blood vessels in the medical images, which is the primary task in clinical applications to support early detection, diagnosis and optimal treatment. In line with the proliferation of imaging modalities, there is an everincreasing demand for the automated vessel analysis systems for which, blood vessel segmentation is the first and most fundamental step. Segmentation is needed for the analysis of these blood vessels at different angles. So, various filters have been proposed to enhance these medical images to overcome the problems while doing segmentation [1].
In particular, a local phase based filter recently introduced by Yitian Zhao et al. [1] seems to be superior to intensity based filters [2]-[4] as it is immune to the intensity in homogeneity and worth to enhance the vessels at different widths. Morphological filters with multiscale Gaussian filters have also shown some interesting results [5]. But the disadvantage with these filters is that, they do not consider the known vessel cross-sectional shape information, and use of an overly long structuring element may cause difficulty in detecting the highly tortuous vessels. To overcome these disadvantages, the proposed model uses Cauchy filter[6] to enhance these medical images. And also the accuracy of the image is increased.
The remainder of this paper is structured as follows. Section 1 deals with related work done over the years. Section 2 deals with the proposed model with Cauchy filter as enhancement filter. Section 3 provides information about Datasets and Evaluation Criteria. Section 4 provides the results of the model. The last section concludes the paper with future scope.
Filters which are used to enhance vessel structures are playing an important role in the vessel segmentation problems. Here, the authors have reviewed the three most influential filters.
Based on Hessian matrix eigen values, this filter is used. For each pixel of a image with intensity f(x), the Hessian matrix can be formed by its 3 second derivatives, and from which two eigenvalues can be computed. The filter is given as
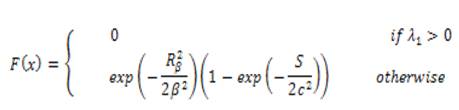
The Isotropic Undecimated Wavelet Transform (IUWT) has recently been used for vessel segmentation. Applied to a signal, c0 = f, subsequent scaling coefficients are calculated by convolution with a filter as follows.
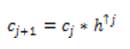
where hₒ= [1, 4, 6, 4, 1] /16 is derived from the cubic Bspline. If f is multidimensional, the filtering can be applied separable in all dimensions. Wavelets are then the difference between two adjacent sets of scaling coefficients, i.e.
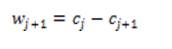
The final set of scaling coefficients is straightforward, and requires only addition and the n wavelet levels
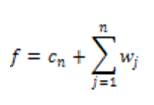
Local phase can measure structural information (e.g. lines and edges) of an image. For imaging applications, local phase uses quadrature filters under the concept of monogenic signals. The response at scale n is given as,
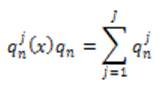
where J is the number of directions under consideration.
By combining the responses from each of the scales, the overall response, P is given below,
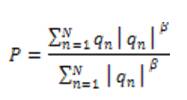
where N is the number of scales and β is the weighting parameter with value ≥ 1.
In practice, the real part of LP, Re (LP), is used as the 'vesselness map'. It has a positive value inside the lines (or vessels) but a negative value in the background, and zero at the edge of the line structures.
The IPACHI model was proposed for the segmentation of objects with irregular boundaries and to integrate hybrid region into the segmentation model. The energy of the IPACHI model is:
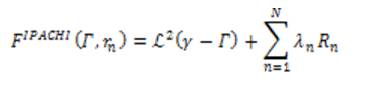
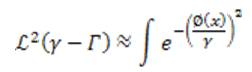
where ℒ² is the 2D Lebesgue measure, Rn is the nth region information, and N is the total number of different region terms. The first term ℒ² is the area of the neighborhood of the edge set.
Here, we consider equation (8) for a large and even number α, which is an approximation of the γ- neighborhood area in a given image u0(x). Different region terms can be used alone (N = 1) or combined (N > 1) for the need of specific applications. For this vessel segmentation application, the authors use the 'vesselness map', v0 of an image and the image intensity, u0 as two distinct region terms to extract vessels of an object with irregular and oscillatory boundaries.
Inspired by different enhancement models [2], [3], the authors propose a novel extension Cauchy PDF as the new kernel of the filter and shows that the new PDF improves the accuracy of the vessel detection. They discovered that the Cauchy PDF shows the vessel patterns better and behaves more flexible than the existing methods. Thus more vessels can be detected that uses the Cauchy function [6].
The Cauchy PDF is defined as:
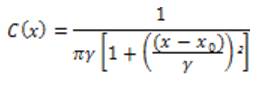
where xₒ is the location parameter and γ is the scale parameter, and these have an effect on the vessels detection. By constructing the filter set using Cauchy kernel, the retinal image is convolved and the maximum response based on the direction of the filter is calculated. The proposed one is to use a local entropy thresholding algorithm that was introduced in [10] to produce the binary images of the Cauchy filtered image. This entropy based thresholding algorithm is more accurate than other competitive methods, because the dependencies between the pixels' intensity of the filtered image allows us to preserve the spatial structure in the thresholding images. Furthermore, combining it with global thresholding, it is assured that the vessels are correctly distinguished from the non-vessels.
The authors have worked with three public retinal image datasets for evaluating the segmentation model. The images in three datasets are the center of the retina. In this section, introduction to these datasets are provided and followed by the evaluation metrics, which are used in these experiments.
40 color images are used in this dataset, obtained in the course of a diabetic retinopathy screening program in Netherland. The images were acquired with a 45 degree field of view. Resolution of an retinal image is 768×584 pixels. The 40 color images is divided into training set and test set, each consisting of 20 images [12].
20 color retinal images are used. Out of 20, 10 of which show evidence of pathology. The digitized slides were captured by a TopCon TRV-50 fundus camera (Topcon, Tokyo, Japan) and the photos were digitized to 605×700 pixels [13].
This dataset comprises of eight ultra-wide field of view retinal angiographic images acquired with an OPTOS P200C camera (Optos PLC, Dunfermline, UK). Four of the images are from an AMD retina, while the other four are from a healthy retina. Each image has a size of 3900×3072 pixel.
The evaluation metrics were employed to evaluate the performance of the competing methods in terms of pixels including, the different performance parameters employed, which are used to measure the quality of reconstruction image:
sensitivity (Se) = tp/(tp + fn),
specificity (Sp) = tn/(tn +fp),
accuracy (Acc) = (tp + tn)/(tp + fp + tn + fn),
The area under a receiver operating characteristic curve,
AUC=(Se+Sp)/2,
precision=tp/(tp+fp),
recall=tp/(tp+fn),
Fmeasure=2 ((precision*recall)/(precision+recall)),
tp, tn, fp and fn indicate the true positive (vessel pixels are correctly detected), true negative (vesselness pixels are correctly detected), false positive (vessel pixels are incorrectly detected), and false negative (vesselness pixels are incorrectly detected), respectively which are used for the proposed model.
In this section, the authors present experiments to evaluate the performance of the proposed model and evaluate the effects of vessel enhancement on the performance across all three datasets. The experiments were performed in Matlab version 2015a (Mathworks, Natick, CA) on a PC 4GB RAM. First, the effect of three different vessel enhancement filters was evaluated which is shown in Figure1 and Figure 2 shows the illustrative results.
Figure 1. Enhancement results produced by the eigenvalue-based method, wavelet-based method and local phase methods, respectively. Three images were randomly chosen from three datasets (one image per dataset). From top to bottom: DRIVE, STARE, and VAMPIRE. (b) Eigenvalue-based enhancement results, (c) Wavelet-based enhancement results, (d) Local phase based enhancement results [1]
Figure 2. Illustrative Results (A) A randomly chosen image from DRIVE dataset (B) Gray sale image of (A). (C)-(F) Enhancement results on (A) by using eigenvalue-based (FR), Cauchy-based, wavelet- based (IUWT), and Local Phase-based (LP) filters respectively (G)-(H) IPACHI and Cauchy segmentation respectively
Table 1 shows the performance of the three different enhancement methods (LP, WL, FR) on the DRIVE, STARE, VAMPIRE datasets, where LP: Local Phase based filter [4]; WL: Wavelet based (IUWT) filter [3]; FR: Frangi's Eigen Value based filter [2]; Sp: specificity; Se: sensitivity; AUC: Area Under Curve; Acc: accuracy.
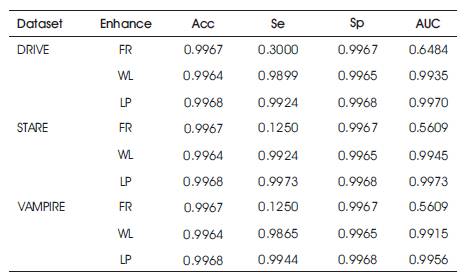
Table 1. Performance Parameters of DRIVE, STARE and VAMPIRE Datasets
The performance parameters of the three datasets which are used commonly has been evaluated and shown in Table 1. The proposed Cauchy filter based segmentation model is the Cauchy segmentation model compared with the IPACHI segmentation model. The results of the proposed model are compared with IPACHI model on the DRIVE, STARE, and VAMPIRE datasets as demonstrated in Table 2. It can be observed from Table 2 that the results of the proposed model in terms of Se, Sp, Acc, AUC, precision, recall, f-measure, g-mean, MSE, and PSNR outperform the competitors in the DRIVE [12], STARE [13] and VAMPIRE datasets.
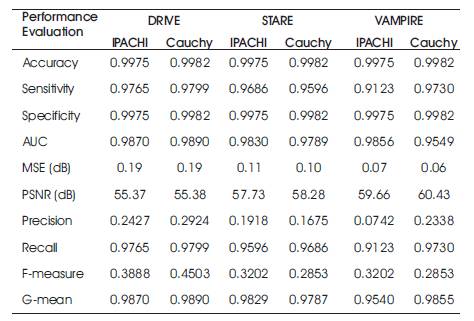
Table 2. Results of the Proposed Model vs IPACHI and Cauchy segmentation on DRIVE, STARE and VAMPIRE Datasets
The proposed model is both effective and efficient for blood vessel segmentation. To emphasize the effectiveness of the model, the authors compare their model with other existing vessel detection methods on two most popular public datasets: DRIVE and STARE.
The VAMPIRE dataset is relatively new and consequently there are relatively only few results in the literature. Table 3 shows the performance of this method and other methods on both the DRIVE and STARE datasets in terms of sensitivity, specifity, accuracy and area under curve. The results have been ordered by the category of the previous methods such as, supervised and unsupervised, in which the proposed method has better performance than the other methods.
The authors propose a new kernel function for the retinal blood vessel filter based segmentation of active contour model for the vessel segmentation problem. This model has been applied to three publicly available retinal datasets images and has shown a good accuracy. The detection of blood vessels is essential and an important step for vessel analysis to perform more advanced analysis, such as diameter measurement and twisted vessels, calculation of the arteriovenous ratio, and diagnostic and prognostic values of eye disease and systematic diseases like stroke, hypertension, etc.
This model was developed by different segmentation methods along with the kernel based Cauchy filter. The Cauchy filter is used to enhance the image and then the IPACHI [1] segmentation is evaluated. The segmentation will separate the vessel and the vesselness images. In conclusion, all in all, from the results, it is deduced that the offered filter with the Cauchy PDF as its kernel is a better choice to detect blood vessels in the digital retinal images. This will be a powerful tool for analyzing vasculature for better management of a wide spectrum of vascularrelated diseases.
This can be extended by extracting Statistical Texture Parameters [11], which are useful in real time pattern recognition applications, like Military and Medical Applications. This model is also suited for segmentation problems in the images of other organs acquired by different imaging techniques such as CT, MRI, and X-ray images.