
Table 1. Properties of GGBS
Carbon Dioxide (CO2) is a byproduct of cement manufacturing process. It is produced in huge quantities while manufacturing Ordinary Portland Cement (OPC). Production of Ordinary Portland Cement Concrete (OPCC) utilizes large quantities of energy and natural resources. Geopolymer Concrete (GPC) came as an alternative to Ordinary Portland Cement Concrete (OPCC). In the present work, silica fume has been used as a replacement to Ground Granulated Blast Furnace Slag (GGBS). This study mainly presents the mix design steps and the mechanical properties of the GPC. The mix design proportions for the GPC were based on trial and error. GGBS from the mix proportions was replaced with Silica Fume in the percentages of 20%, 40%, 50%, 60%, 80%, and 100%. The mechanical properties like compressive strength, split tensile strength, flexural rigidity, and modulus of elasticity were studied for all the replacements. It was shown that the specimens with only GGBS as source material are high in strength. It was observed that the properties like compressive strength, split tensile strength, flexural strength showed a significant reduction with the increase in silica fume quantity. A delay in the setting time of GPC was observed with the increase in Silica Fume content.
Concrete is an affordable and durable material. The concrete is used widely in the construction industry because it is easier to mix, transport, and place. Considerable quantity of cement is used in the production of concrete. As the emissions of carbon dioxide are very high during the manufacture of Ordinary Portland cement, researchers have tried to find more sustainable alternative to Portland cement. Cement production contributes to 7% of the global carbon dioxide emission. Dr. Joseph Davidovits coined the term Geopolymer to refer binders made from alumino-slicate materials like Fly Ash, Rice husk ash, GGBS, and Metakaolin.
Geopolymer concrete contains a source material and an alkali activator. Source materials in GPC include fly ash, GGBS, silica fume, and metakaolin. Alkali activator is a combination of either sodium hydroxide and sodium silicate or potassium hydroxide and potassium silicate. The alkali activator has to be chosen and the hydroxide solution must have a specific molarity. The source materials used in the preparation of GPC are industrial byproducts. These byproducts are also used as mineral ad- mixtures in the preparation of OPCC. Their utilization as admixtures in OPCC is very less. So these byproducts occupy certain areas as storage places.
GPC does not require cement for preparation and uses only these industrial byproducts as source materials. One of the benefits of using GPC is that it does not require water for curing. This helps in utilization of water very sparingly as it is a very scarce natural resource. GPC requires ambient curing or sometimes heat curing based on the source material chosen. Since no cement will be used in GPC, there is also slight reduction in the production of carbon footprint.
Lloyd and Rangan (2010) have studied fly ash-based geopolymer concrete precast box culverts. Naidu, Adiseshu, and Satayanarayana (2012) have attempted to study strength properties of geopolymer concrete using low calcium fly ash replacing with slag in five different percentages. Vijai, Kumutha, and Vishnuram (2013) have presented the experimental investigation on the mechanical properties of geopolymer concrete composites containing 90% Fly ash, 10% Ordinary Portland Cement, and alkaline liquid. Vora and Dave (2013) investigated various parameters like ratio of alkaline liquid to fly ash, concentration of sodium hydroxide, ratio of sodium silicate to sodium hydroxide, curing time, curing temperature, dosage of super plasticiser, rest period, and additional water content in the mix. Nath and Sarker (2014) aimed to prepare a low calcium fly ash based geopolymer at ambient curing. They used GGBS to achieve the required workability, setting time, and compressive strength.
An attempt was made by Ramujee and Potharaju (2013) for developing a mix design for different grades of concrete like low, medium, and higher. They used Fly ash as a source material and suggested optimized mix proportions of different grades of concrete. A study on the incorporation of GGBS in GPC was done by Supraja and Rao (2011). They used sodium hydroxide as alkaline solution and prepared specimens with different molarities of sodium hydroxide. They have observed that with the increase in the molarity of the alkaline liquid, compressive strength also increased. Adam and Horianto (2014) experimented on the heat curing duration of Fly ash based GPC. They exposed GPC to the temperatures of 80 oC, 100 oC, and 120 oC for periods of 4, 6, and 20 hours durations. They observed that GPC exposed to 120 oC for a period of 20 hours has highest compressive strength. The properties like setting time, strength development, workability, and drying shrinkage of high calcium fly ash based mortar was studied by Chindaprasirt, Chareerat, Hatanaka, and Cao (2010). They observed a decrease in the setting time of mortar with the increase in the fineness of fly ash used. Also the characteristics like drying shrinkage, flow, and strength were improved with finer fly ash. According to Kishanrao (2013), the compressive strength of GPC with fly ash and GGBS decreased when exposed to temparatures varying between 100 oC and 500 oC.
Parthiban, Saravanarajamohan, Shobana, and Bhaskar (2013) observed the increase in compressive strength of fly ash based GPC with the increase of GGBS as a replacement. The behaviour of class F fly ash based GPC under acidic environment was studied by Gopal and Kiran (2013). They used three different acidic solutions in the study and determined the loss of compressive strength and mass of the cubes. It was observed that exposure to HCL acidic solution had greater weight loss than other solutions. The study on compressive strengths of various alkali to binder ratios was done by Joshi and Kadu (2012). The optimum compressive strength was seen in the ratio of 0.3 under oven drying of 75 oC for a period of 48 hours. The fracture behaviour of fly ash based GPC was discussed by Sarker, Haque, and Ramgolam (2013). They concluded that GPC has more tensile strength than OPCC and also the difference in the fracture behaviour is due to higher bond strength of GPC over OPCC. Kong and Sanjayan (2010) have presented the effect of elevated temperatures on the Geopolymer paste, mortar, and concrete. They exposed the specimens to varying temperatures between 100 oC and 800 oC and found that the specimens with aggregate size greater than 10 mm have more stable behaviour.
The behaviour of fly ash based GPC with varying fine aggregate to total aggregate ratios were studied by Joseph and Mathew (2012). The GPC with 70% by volume of fine aggregate to total aggregate performed better than other specimens. Bhavsar, Talavia, Suthar, Amin, and Parmar (2014) experimented on the workability characteristics of fly ash based GPC. Usage of silica fume decreased the workability and usage of superplasticizer increased the workability. A comparative study on the emissions of carbon gases and costs between GPC and OPCC was done by McLellan, Williams, Lay, Riessen, and Corder (2011). They have done the study on financial and environmental costs and concluded that the source location, mode of transport, and energy location determine the variation of costs. A feasibility study on using coarser bottom ash and GGBS as a replacement to flyash was done by Mathew, Sudhakar, and Natarajan (2013). They concluded that Bottom ash- GGBS based concrete has lesser strength than flyash based GPC. The durability of flyash based GPC in marine environment was experimented and studied by Reddy, Edouard, Sobhan, and Tipnis (2011). A comparative study between GPC and OPCC was done by exposing the specimens to marine conditions. It was observed that GPC performed better than OPCC under chloride attack.
Chakraborty, Mallik, Del, and Pal (2012) have studied the durability of flyash based GPC when the specimens were immersed in 10% sodium sulphate. A reduction in the residual strength was observed with the increase in the duration of exposure. The potential of geopolymer concrete towards the green buildings was studied and presented by Komnitsas (2011). Suggested that more work should be done in the area of commercial applications of GPC. Rajarajeswari and Dhinakaran (2014) attempted to study the silica fume based GPC with varying alkaline liquid. They concluded that replacement of cement with Silica Fume reduced the compressive strength of concrete. Addition of silica fume with fly ash percentages in the range of 2.5–5% was studied by Dutta, Thokchom, Ghosh, and Ghosh (2010) on concrete and mortar. Addition of silica fume increased the compressive strength of concrete and reduced the mortar strength. Alekhya and Aravindan (2014) studied the experimental investigations on geopolymer concrete and discussed its ethical issues.
The traditional method of manufacturing concrete has put the advancement of this very important construction material in slow pace. The huge increase in demand from housing industry due to the population explosion has increased the gap between supply and demand. In order to meet the increasing demand, researchers have been trying to find out more sustainable alternative for construction material. Research has been undertaken in various parts of the world to manufacture concrete using industrial waste products like fly ash, Ground Granulated Blast furnace Slag (hereafter referred as GGBS), rice husk ash, metakaolin, and silica fume.
The main objective of this work is to prepare a mix design procedure and study the mechanical properties of GGBS based geopolymer concrete by incorporating silica fume at varying proportions to GGBS. In this study, GGBS is replaced with silica fume in seven different proportions. For each proportion, the mechanical properties like compressive strength, split tensile strength, modulus of rupture (flexural strength), modulus of elasticity, and carbonation tests were done.
4.1.1 GGBS
Ground Granulated Blast furnace Slag is a by-product of steel or iron manufacturing industries. GGBS is formed by quenching the molten slag produced from a blast furnace. It is a granular product, which is dried and powdered. The chemical composition of the GGBS depends upon the type of manufacturing plant and is shown in Table 2. The major components of GGBS are Silica and oxides of aluminium. The GGBS used in this study is from Vizag steel plant, Visakhapatnam, supplied by SVSS Enterprises Pvt. Ltd, Autonagar, Visakhapatnam. The properties like specific gravity and fineness are shown in Table 1.

Table 1. Properties of GGBS

Table 2. Composition of GGBS
4.1.2 Silica Fume
Silica Fume is the by-product obtained from the silicon production industries. It consists of very fine spherical particles of silca fume widely used in blended concretes for specialised conditions. The Silica Fume used in this work was supplied from BTL industries, Autonagar, Visakhapatnam. Table 3 shows the properties of silica fume and the compostion is shown in Table 4.

Table 3. Properties of Silica Fume

Table 4. Composition of Silica Fume
4.1.3 Fine Aggregate The fine aggregate used in this study is clean river sand, purchased from a nearby quarry in Visakhapatnam. The following tests were carried out on Fine aggregate as per IS 2386-1968 (Part 3) and its properties were tabulated in Table 5.
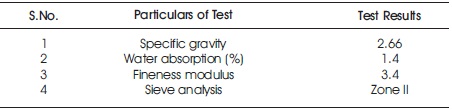
Table 5. Properties of Fine Aggregate
4.1.4 Coarse Aggregate
The coarse aggregate used in the present study is crushed stone of size 20 mm and down from Visakhapatnam and the following tests were done on the coarse aggregate as per IS 2386-1968 (Part-3) and the properties were tabulated in Table 6.
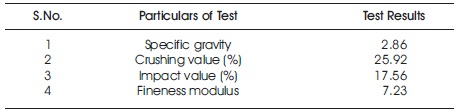
Table 6. Properties of Coarse Aggregate
4.1.5 Alkaline Liquid
The alkaline liquid used in this study was a combination of Sodium Hydroxide (NaOH) and Sodium Silicate (Na2SiO3). Sodium hydroxide is available in the form of flakes is shown in Figure 1 and sodium silicate shown in Figure 2 is available in the form of liquid. Sodium Hydroxide concentration of 10M was adopted. The sodium hydroxide solution must be prepared 24 hours prior to casting and sodium silicate must be mixed with sodium hydroxide solution 1 hour prior to casting. The ratio between sodium hydroxide to sodium silicate was maintained as 2.5.

Figure 1. Sodium Hydroxide (NaOH) Flakes used in the Study

Figure 2. Alkaline Liquid used in the Study
4.1.6 Size of the Specimens
Cube specimens used for testing were of size 100 x 100 x 100 mm3. The size of the cylindrical specimens were 150 mm diameter and 300 mm length, generally their length are twice the size of their diameter. The beam specimens used were 500 mm length and the dimensions of the cross section were 100 mm x 100 mm. The specimen dimensions are according to IS: 10086-1982.
4.1.7 Curing
After de-moulding, the concrete specimens were kept for curing. As there will be no heat of hydration in GPC, the specimens were not water cured. There are two types of curing for GPC, namely ambient curing and heat curing. In ambient curing, the specimens are cured at ambient temperature. In heat curing, the specimens are cured at a specified temperature in oven by covering the specimens with polythene covers in order to restrict the humidity. In this work, ambient curing was done in practical point of view. Specimens were cured for 7 and 28 days at ambient temperature.
4.1.8 Tests on Specimens
After curing, the cube specimens were tested for compressive strength and cylinder specimens for split tensile strength using compression testing machine of 200 ton capacity at the rate of 400 kg/min loading. For flexural strength beam specimens were tested on a universal testing machine of capacity 200 tons. Modulus of elasticity for the cylinder specimens were tested with a compressometer on a compression testing machine. Carbonation test was carried on the specimens using phenolphthalein indicator. The above tests were carried on the specimens for 7 and 28 days as per Indian Standards.
There is no standard mix design procedure for GPC. To obtain the mix proportions it is required to adopt trial and error method.
The design mix used for this study was 1: 1.35: 2.03. The water to source material (GGBS and silica fume) is 0.41. To study the mechanical properties of GGBS based GPC 7, different proportions were prepared with different percentages of silica fume by weight as shown in Table 7. GGBS in the concrete was replaced with silica fume from 0- 100% by weight (0, 20, 40, 50, 60, 80, and 100). In each mixture, 6 specimens were prepared. 3 were tested for 7 days and 3 for 28 days as shown in Table 8.
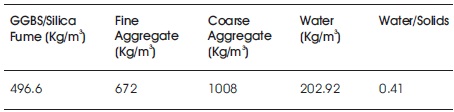
Table 7. Mix Proportions

Table 8. Nomenclature for Mix Proportions adopted in the Experimental Program
From Figure 3, it is observed that GGBS based GPC has high early strength of 62.6 MPa at 7 days of ambient curing, which is 40% more than required. The compressive strength is decreased with the increase in silica fume replacing GGBS. From the graph it is observed that at 28 days of ambient curing, the compressive strength of GPC at 0% replacement is 68.33 MPa and 100% replacement is 13.33 MPa.

Figure 3. Variation in the Compressive Strength of GPC at 7 and 28 Days of Ambient Curing
The split tensile strength decreased with the increase in silica fume replacing GGBS. Reduction in split tensile strength at 7 days ambient curing is continuous and significant from 0% to 50% of replacement, which is from 3.57 MPa to 1.73 MPa. However from 50% to 100% replacement, the reduction is gradual from 1.73 MPa to 0.85 MPa. The variation of spit tensile strength values are displayed in Figure 4.
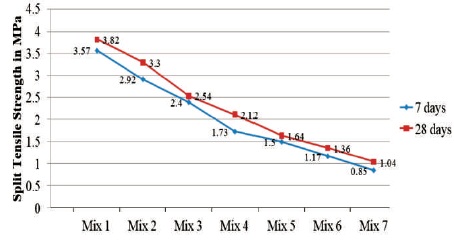
Figure 4. Variation in the Split Tensile Strength of GPC at 7 and 28 Days of Ambient Curing
Figure 5 displays the variation in flexural strength of the specimens. The increase in flexural strength between respective mixes from 7 to 28 days of ambient curing is very less. Reduction in flexural strength is continuous and significant from 0% to 40% of replacement, which is from 4.13 MPa to 2.56 MPa. However from 40% to 100% replacement, the reduction is gradual from 2.56 MPa to 1.36 MPa.
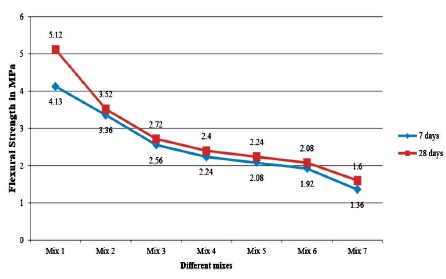
Figure 5. Variation in the Flexural Strength of GPC at 7 and 28 Days
From Figures 6 and 7, it is observed that for 0% replacement with silica fume, the value of Young’s modulus is 9733.33 MPa and with 100%, the value is 4275 MPa. There is a significant decrease in the value of modulus of elasticity up to 50% replacement, which is 7566.6 MPa. The value decreased gradually from 60% replacement, 7500.6 MPa. However, the final value at 100% is low when compared with other replacements.
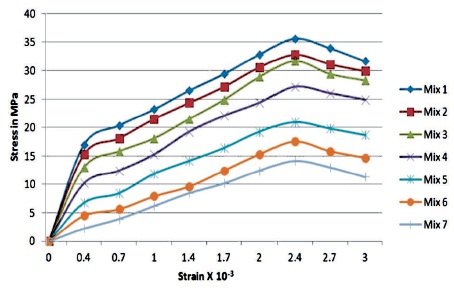
Figure 6. Variation in Stress-Strain

Figure 7. Variation in Modulus of Elasticity
The results show that there is no carbonation effect on the GPC at 28 days of ambient curing. However, there is a slight reaction of carbon dioxide on the surface of the cube specimens. But there is no carbonation effect inside the surface of GPC.
From Figure 8, it is observed that for 0% replacement with silica fume, the value of Young’s modulus is 9733.33 MPa and with 100%, the value is 4275 MPa. There is a significant decrease in the value of modulus of elasticity up to 50% replacement, which is 7566.6 MPa. The value decreased gradually from 60% replacement, 7500.6 MPa.
The following conclusions are made on the results.