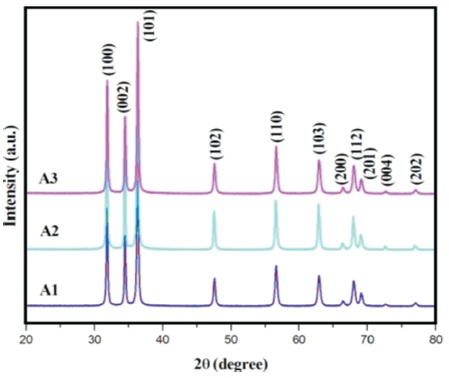
Figure 1. XRD Patterns of the As-Synthesized Samples
Zinc oxide (ZnO) nanostructures have been synthesized using zinc acetate dehydrate and deionised water as precursors by the hydrothermal method. To investigate the morphology and size of the ZnO nanostructures, different concentrations of organic ligand hydrazine hydrate were added as a passivating agent to the precursor solution. The crystal structure of the synthesized samples were analysed by X-ray diffractometer. The influence of variation of hydrazine hydrate concentration in the synthesized samples has been investigated from the field emission scanning electron microscope images. From the diffuse reflectance spectroscopy studies, the optical absorption and band gap of the samples were determined. The samples were examined for morphology and size dependent photocatalytic activity against the degradation of methylene blue organic dye under visible light irradiation.
In recent years, remarkable attention has been given to nanostructured materials by researchers due to their attractive performance in optics, photonics and electronics (Baruah & Dutta, 2009). These materials possess high surface to volume ratio, enhanced optical and electronic properties in comparison with bulk counterpart. The properties of nanomaterials can be tailored by changing their size and morphology (Madathil et al., 2007). In today's world, the environmental pollution is a major problem which is mainly due to the various organic and inorganic pollutants, chlorinated aromatic compounds, etc. Therefore, various methods have been adopted for the remediation of pollutants. The conventional methods were employed to reduce the organic pollutants but they do not destroy pollutants entirely (Ozdemir et al., 2004). At present, nanomaterials have an important role in the photocatalytic applications. The most promising technology is photocatalytic oxidation and reduction, which occurs when the nanomaterials are used as photocatalyst for the degradation of pollutants (Srinivasan et al., 2019). Nanomaterials, especially metal oxide semiconductors are used as photocatalyst for the complete oxidation of hazardous compounds (Faisal et al., 2007). In photocatalysis, ZnO has been recognized as a benchmark catalyst (Ismail et al., 2005; Srinivasan et al., 2019). Also, for the degradation of any organics in aqueous solution, ZnO is an alternative photocatalyst to TiO (Gouvea et al., 2000). 2 Different methods have been employed to synthesize ZnO nanostructures such as sol-gel, co-precipitation, chemical vapour deposition, thermal decomposition, hydrothermal method. From the methods mentioned above, hydrothermal method is one of the major well-developed synthesis methods for ZnO micro and nanostructures because of the reaction process that occurs under a sealed environment (Lincot, 2010). Du et al. (2006) reported the solvothermal synthesis of ZnTe nanorod bundles in a mixed solvent of ethylenediamine and hydrazine hydrate. Different weight percentage of hydrazine hydrate has been used as solvent in the solvothermal synthesis of hexagonal ZnO nanorods (Zhou et al., 2007). Lin et al. (2013) reported the solvothermal synthesis of ZnO microwires and nanowires using a mixture of ethylenediamine with deionized water under 4:1 ratio and hydrazine hydrate with deionized water under 4:1 ratio as solvents with the precursor of Zn foil respectively. Based on the literature review, the controlled morphology of ZnO plays vital role in the environmental remediation process. In the present work, tetrapod and dumb-bell shaped ZnO structures were prepared via hydrothermal method by varying the amount of passivating agent. Under the visible light irradiation, the photocatalytic activity of the samples has been performed against the degradation of methylene blue organic dye.
All chemical reagents of analytical grade were used for the synthesis of ZnO nanorods. In the typical synthesis, the required amount of zinc acetate dehydrate has been dissolved in deionized water in three separate beakers for 30 minutes under vigorous stirring. Three different amount of hydrazine hydrate solution such as 0.4 ml, 0.6 ml and 0.8 ml has been added into the precursor solution. The pH of the solution has been adjusted to 12 by adding NaOH drop by drop and magnetically stirred for 30 minutes. Finally, the solution has been transferred to a Teflon lined autoclave and the reaction has been carried out for 12 hours under 200 0C. After gradually cooling to room temperature, the precipitate has been collected from the autoclave and washed several times with deionized water and ethanol. The final product has been obtained by drying the synthesized sample in a hot air oven at 80 0C. The samples were labelled as A1, A2 and A3 for the 0.4 ml, 0.6 ml and 0.8 ml hydrazine hydrate solution respectively.
X-ray diffraction (XRD) pattern recorded for the synthesized sample is presented in Figure 1. The diffraction patterns of the samples were compared with JCPDS data (card no: 75-1526) and indexed, which confirms the hexagonal wurtzite structure of ZnO. Absence of any other impurity peaks in the XRD patterns indicates the purity of the samples.

Figure 1. XRD Patterns of the As-Synthesized Samples
Figure 2 shows the field emission scanning electron microscopy (FE-SEM) images of the ZnO samples. The tetrapod morphology of ZnO has been obtained for the samples A1 and A2 as shown in Figure 2(a) and 2(b). In the tetrapod structures, each arm has different length and diameter but with a hexaognal cross section at their ends. This is in well agreement with the hexaognal phase of ZnO confirmed by the X-ray diffraction analysis. Figure 2(c) shows the dumb-bell shaped ZnO structures for the sample A3. By increasing the amount of hydrazine hydrate solution from 0.4 ml to 0.8 ml, the modified morphology and size of the ZnO structures has been observed.
Hydrazine hydrate has been readily reacting with zinc acetate to form a slurry-like precipitate of hydrazine zinc complex (Zn(N2H4)2) 2+. The hydrazine complex is acting both as an organic ligand and as a capping agent to yield onedimensional nanostructure (Bhat, 2008). During the solvothermal synthesis, the presence of Zn(OH)4 2+ in the hydrazine hydrate solutions will be helpful for the growth of ZnO structures along the c-axis and also prevent the radial growth of the structures. The possible reaction mechanism for the formation of ZnO nanostructures may be explained as follows (Zhou et al., 2007).
The red shifted optical absorption in the visible region is noticed for the synthesized samples with respect to the bulk ZnO (387 nm) from UV-vis diffuse reflectance spectrometer and is shown in Figure 3(a). The optical absorption is always associated with its band gap energy (Faisal et al., 2013). The band gap of the samples is estimated by extrapolation 2 of the linear curve obtained by plotting (αhγ2) versus hg and the calculated values of bandgap energy for the samples are as shown in Figure 3(b) (Omidi et al., 2014). From the obtained results, the energy gap of all the samples has small value than the commercially available ZnO which indicates that they could be better in the visible light active photocatalysis.
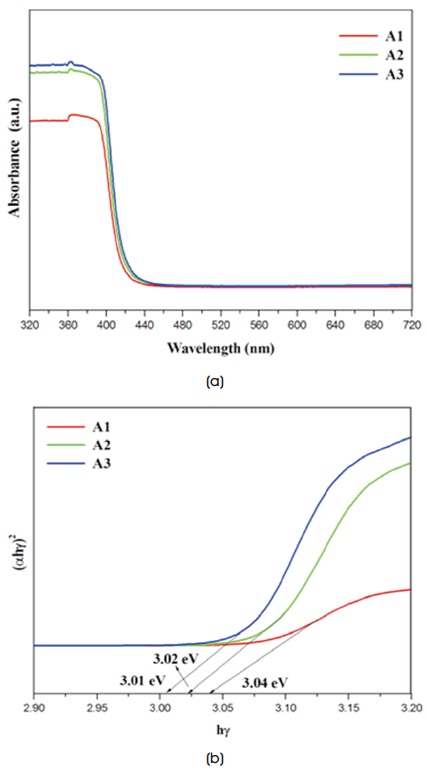
Figure 3. (a) Diffuse Reflectance Spectra, (b) Tauc Plot of As-Synthesised ZnO Samples 4
The photodegradation of methylene blue dye has been noticed as 90.86, 92.95 and 94.93% by the photocatalysts prepared with varying hydrazine hydrate solution amounts of 0.4 ml, 0.6 ml and 0.8 ml, respectively and is shown in Figure 4. From the obtained results, the photocatalyst A3 with dumb-bell shape exhibited the highest photocatalytic activity followed by the photocatalysts A2 and A1. The process of photodegradation of methylene blue dye can be explained as follows. The generation of excitons (electron-hole pair) in ZnO is caused by the irradiation of light onto the photocatalysts. Superoxide anion radicals are formed when the photo induced electrons in the conduction band of ZnO interact with oxygen molecules adsorbed on ZnO. Similarly, water dissociation causes the holes in the valence band of ZnO to produce hydroxyl radicals (Srinivasan et al., 2019). Furthermore, after the complete degradation process, highly reactive hydroxyl radicals and superoxide radicals can react with the organic dye methylene blue to produce harmless products (Sahoo et al., 2015; Srinivasan et al., 2019).
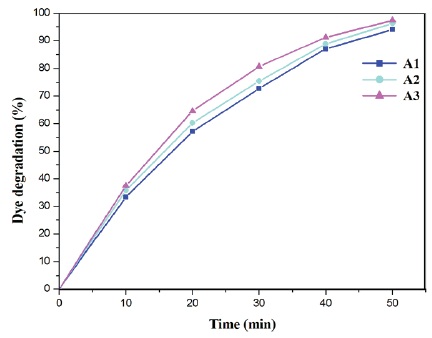
Figure 4. Plot of Dye Degradation (%) as a Function of Irradiation Time
Hydrazine hydrate passivated ZnO nanostructures were successfully synthesized by hydrothermal method. Varying the amount of hydrazine hydrate solution has great influence in the morphology and the size of the synthesized samples. Hydrazine hydrate plays a significant role in the synthesis of ZnO nanostructures. A possible mechanism has been proposed to investigate the formation of ZnO structures. From the optical studies, the red shifted absorption and band gap were noticed for the assynthesized samples. Then, the feasibility of the visible light catalytic activity of the photocatalysts against the degradation of methylene blue dye has been discussed. Among the three samples, the dumb-bell shaped photocatalyst A3 showed enhanced catalytic activity.
The authors would like to thank the Management, Principal, Vice Principal and HOD Physics, SRM Valliammai Engineering College, Kattankulathur, Tamil Nadu, India, for providing the research facilities. The authors are thankful to the Management, Principal, Head of the Department of Science & Humanities and Prof. R. Premanand, Sri Sai Ram Engineering College, Chennai, Tamil Nadu, India, for their encouragement and support. Also, the authors acknowledge Nanotechnology Research Centre, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India for their help to utilize characterization facilities in the laboratory.