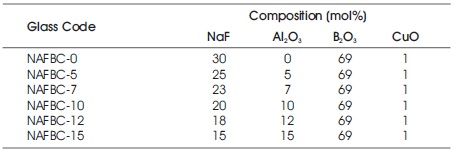
Table 1. Glass Composition
Sodium fluoro alumino borate glasses with composition (30-x) NaF-xAl2O3-69B2O3 -1CuO (where x=0, 5, 7, 10, 12, 15 mol %) were prepared by melt quenching method in which Cu2+ ions are acting as spin probe. EPR spectra were recorded at X - band frequencies with 100 kHz field modulation at room temperature and its resonance peaks shows Cu2+ ions characteristics. From calculated spin Hamiltonian parameters, it has been observed that g∥ >g⊥ >ge (ge =2.0023 for free electron) and A∥ >A⊥ indicated that the site around transition metal ion (TM) i.e., Cu2+ ion is tetragonally distorted octahedral and its ground state is dx2-y2 orbital ( 2B1G state). The number of unpaired Cu2+ ion spins (Ni ) participating in resonance and the paramagnetic susceptibility (χ) were also evaluated. Glass composition is also a important factor and its effect shows the variation in spin Hamiltonian parameters. A middle broad absorption ( 2B1g → 2B2g) band is observed at 770 nm in the optical spectra. Calculated bonding coefficient revealed the bonding nature between TM ion and its ligand.
Transition metal (TM) ions doped oxy-fluoride glasses have been investigated widely because of their important applications in IR transmission components, amplification and 3D display. Especially, the glassy materials are efficient hosts for lasing materials. Compared to all other glass materials, the transparent oxyfluoride glasses containing Cu2+ ions have been given a special attention due to their excellent optical and magnetic properties (Bandyopadhyay, 1981; Sands, 1955; Sekhar et al., 2018; Thulasiramudu & Buddhudu, 2006). Among all fluorides, sodium fluoride has high solubility, good chemical stability (Shareefuddin et al., 1996). Al2O3 is not a glass former, whereas B2O3 readily form glasses by itself. Al2O3 does not fulfill the requirements of glass former. It cannot frame a three-dimensional network of Al2O3 triangles. But boric oxide has three-fold coordination. The Al3+ ion ranges between those particles which prefer to be six-fold or octahedral coordination. These combinations will create considerable changes in the optical and electrical characteristics of the prepared glasses.
Through Electron Paramagnetic Resonance (EPR) spectro scopy one can get information regarding the structural and dynamic properties of materials. TM ions are embedded into the glassy network to get EPR spectra. The EPR spectra inturn reveals about the site symmetry that is present around the TM ions which is quite useful in understanding the basic structure of the glass (Ahmed & Shareefuddin, 2019; Ramadevudu et al., 2000). Many researchers focused mainly on oxide glasses. The work on oxyfluoride glasses is meager and hence we paid attention to study the oxyfluoride glasses behavior through EPR and optical examinations.
Ahmad et al. (2019) studied the effect of Al2O3 on strontium borate glasses using FTIR, EPR and Raman experimental techniques and concluded that the addition of Al2O3 influences the borate network by creating AlO4 units. Copper doped alumina bismuth borate glasses were characterised with XRD, optical absorption, EPR and FTIR techniques by Phani et al. (2019). XRD confirmed the amorphous nature; spin Hamiltonian parameters were evaluated from EPR spectra and concluded that the Cu2+ ions are in tetragonally distorted octahedral sites with ground state (2B1g) dx2-y2. Formation of BO3, BO4 and AlO4 units in the borate network were observed from FTIR spectra. Abd El-Moneim (2019) studied the effect of composition on alkali fluoride and oxyfluoride glasses by correlating some of the physical properties like Poisson's ratio, packing density, etc. Different transition metal ion doped NaF-B2O3 glasses were characterised by Murthy et al. (2000) with dielectric measurements. Quantum mechanical tunnelling model is used to explain AC conductivity of NaF-B2O3 glasses. Franco et al. (2019) prepared the glasses with composition NaPO3–Sb2O3–CuO and characterised with XRD, EPR and optical absorption to study the structural modifications in the glass network and ligand field near Cu2+ ions. Many other researchers (Bale & Rahman, 2013; Sastry & Rao, 2014; Singh et al., 2013; SivaRamaiah & LakshmanaRao, 2013; Vedeanu et al., 2012) prepared the glass samples with various compositions doped with copper, explaining about environment around transition metal ion using the optical and EPR spectra.
In this paper, we report the effect of incorporating NaF and Al2O3 into B2O3 network.
Melt-quenching method is employed for the glass fabrication. Table 1 gives the details of the glass composition. Alumino sodium fluoroborate glass (30−x) NaF-xAl2O3-69B2O3 -1CuO (NFABC) were prepared by conventional melt quenching technique. In this the concentration of Al2O3 is varied from 0 to 15 mol% in the place of NaF concentration, which is decreased from 30 to 15 mol% and to this 1 mol% of CuO has been added as paramagnetic probe. In the preparation of these glass samples the analar grade boric acid (H3BO3 - 99% purity), sodium fluoride (NaF- 99% purity), aluminum oxide (Al2O3 - 99% purity), and copper oxide (CuO- 99% purity) of Aldrich/ Merck make were used as the starting materials. For a given composition, the concentration in mole% of each chemical is calculated and the raw chemicals were weighed in digital monopan balance. All the chemicals forming the glass composition are finely ground to achieve homogeneity. The glass composition is taken in platinum crucible and heated to melt in an electrically heated furnace at 1000 °C. After obtaining homogeneous mixtures the melt were quenched at 200 °C and kept at same temperature to mechanical stresses. The glass compositions with appropriate glass code are given in Table 1.

Table 1. Glass Composition
Philips X-ray diffractometer is used to scan the 2θ range from 10 to 80 degrees. Peak free X-ray diffractograms of NAFBC glass samples confirmed the amorphous nature. Absorption spectra of NAFBC samples recorded at room temperature in the wavelength 300–1200 nm on Shimadzu UV-1800 model spectrophotometer. EPR Spectra of Cu2+ ions are recorded using JEOL-1X-EPR operating at X-band with field modulation of 100 kHz. Uncertainties in the measurement of “g” and “A” values were about ±2x10-3 cm-1 and ±2x10−4 cm−1 , respectively.
Cu2+ ion is in its oxidation state, and the copper ion will have electronic configuration [Ar] 3d⁹. When the external magnetic field is applied, this degeneracy is removed and the transition occurs between the two energy levels in accordance with the resonance condition, hn = gβH. The nuclear spin (I) and electronic spin (S) of the Cu2+ ion are 3/2 and 1/2 respectively. The interaction between the nuclear magnetic moment and electronic magnetic moment (both spin and orbital) results in the hyperfine structure seen in the EPR. Thus, each fine structure splits into (2I+1) components (Abragam & Bleaney, 1970). In the case of Cu2+ ion (3d9 configuration) the cubic field (six negative charges placed on the axes at equal distance from the metal ion) splits the eg and t2g orbitals where eg orbitals will have more energy than t2g orbitals (Jorgensen, 1955).
In the case of tetragonal symmetry, where the distance of the negative charges on the z axes from the origin is longer than that in the equatorial plane, the degeneracy of the 3d orbitals of d9 will be further removed. The EPR spectrum of Cu2+ ion in most of the oxide glasses is distinctive and easily recognized based on the principal 'g' values and four line hyperfine splittings. In majority of oxide glasses containing Cu2+ complexes, the coordination of Cu2+ ion is that of a tetragonally elongated octahedral.
The EPR spectra of NAFBC glasses are shown in Figure 1. The EPR Spectra of Cu2+ ions is recognized in the form of four parallel and four perpendicular hyperfine splitting components. However we noticed only three well resolved parallel components. No perpendicular components are resolved because the spectrum can be recognized on the basis of four hyperfine line splitting due to 63Cu and 65Cu. But the isotropic splitting are not resolved owing to the nearly identical nuclear moments.
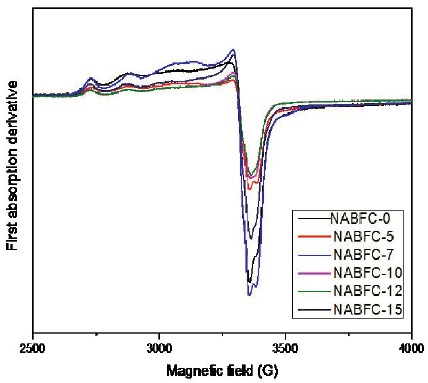
Figure 1. EPR Spectra of (30−x) NaF-xAl2O3 -69B2O31CuO Glass System
The EPR spectra NAFBC samples are similar to those reported earlier (Zamyatin et al., 2018). For NAFBC glass system an axial spin Hamiltonian is employed (Abragam & Bleaney, 1970).
The spin Hamiltonian parameters are evaluated and are presented in Table 2. The g∥ values varied between 2.3296 for NAFBC-7 sample and 2.3737 for NAFBC-12 and g⊥ values varied between 2.076 for NAFBC-0 and 2.128 for NAFBC-12. NAFBC-12 has got maximum values. From the Table 2 it was observed that, g∥ >g⊥ >ge (ge = 2.0023) and A∥ >A⊥ and hence it is concluded that dx2-y2 - orbital (2B1g state) being the ground state of Cu2+ ions and are octahedral sites with tetragonal distortion.
Table 2. Spin Hamiltonian Parameters, Optical Absorption Bands of (30−x) NaF-xAl2O3 -69B2O3-1CuO Glass Systems
It can be observed from Table 2 that the variation of g and A∥ with Al2 O3 content is found to be non-linear. Variation in g∥ and A∥ values may be associated with the change in the environment around Cu2+ ion, i.e., in the ligand field strength at the site of Cu2+.
The number of spins (N) and susceptibility (χ) values were evaluated for NAFBC samples and presented in Table 3 (Aschcroft & Mermin, 2001; Ashok et al., 2018; Shareefuddin et al., 1996; Weil & Bolton, 2007).
The variation in N and χ with glass composition is shown in Figure 3. It is observed that both are varying non linearly and showing opposite behaviour. These variations confirms that the glass composition effects the glass network around the transition metal ions.
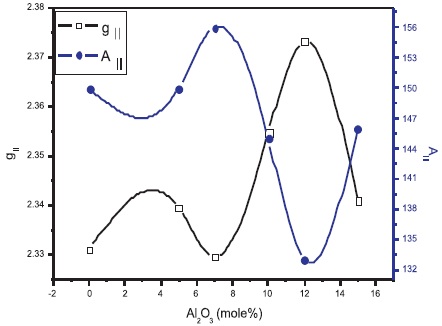
Figure 2. Variation of gII and AII with Al2O3 Content
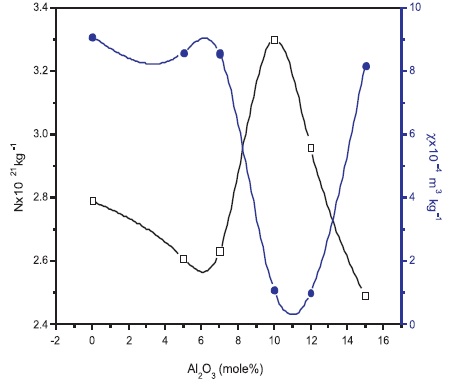
Figure 3. Variation of N and χ with Al2O3 Content
Interestingly, the N value with increasing Al2O3 concentration in the glass has shown inflections. This variation is due to slight modification of boron network by Al2O3 .
A broad absorption band is observed in near IR region in UV visible spectra of NAFBC glasses (Figure 4) which is the characteristic peak of Cu2+ ions. The observed peak positions of the optical absorption spectra of the glasses are listed in Table 2 which are assigned to the 2B1g → 2B2g transition of Cu2+ ions (Ahmed et al., 2018). With increasing Al2O3 content, the absorption peak is found to vary within ±10 nm from 770 nm. The ΔExy values are varied between 12820 for NAFBC-10 and 12936 for NAFBC-5.
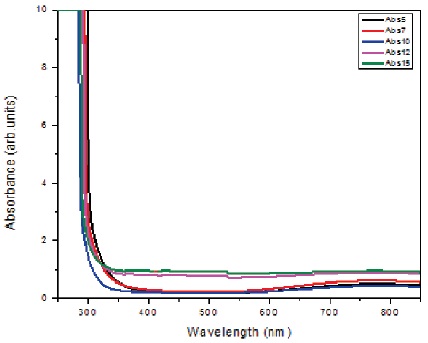
Figure 4. Optical Spectra of (30−x)NaF-xAl2O3-69B2O3-1CuO Glass System
The observed optical peak in IR region (ΔExy ) used to calculate the bond parameters.
α2 values varied between 0.82 and 0.84, β12 values varied between 0.76 and 0.86 & β2 values varied between 0.91 and 0.93. The calculated values as shown in Table 3 of α2 and α12 indicated in plane σ -bonding as well as in plane π-bonding are moderately ionic and while out of plane π- bonding, β2 seems to be ionic (Sekhar et al., 2016). The cupric ion is a network modifier along with NaF and Al2O3 in the B2O3 glass network.
The competition between the glass former cations and cupric ion in attracting neighboring alone pairs of intervening oxygen ions can be known from β12 . The value of β12 strongly depends on network former.
The normalized covalency (Wei et al., 2005) of Cu2+ -O in plane bonding σ and π symmetries (resp., tσ, and tπ) can σ p be expressed interms of bonding coefficients α2 and β2 .
(30−x)NaF-xAl2O3-69B2O3 -1CuO (where x=0, 5, 7, 10, 12 & 15 mol%) glasses were prepared using melt quenching method and characterised with XRD, optical absorption and EPR spectroscopic techniques. Non-crystalline nature is confirmed by observing peak free spectrograms of XRD. Optical absorption spectra showed a broad absorption band at Infrared region, which was attributed to 2B1g → 2B2g transition. EPR spectra of NAFBC glasses exhibited three parallel lines in low field region and fourth component coincided partially with the perpendicular ones in high field region. The spin Hamiltonian and bonding parameters were calculated by harmonizing the EPR and optical absorption spectra. The estimated g∥ and g⊥ satisfied the relationships g∥ >g⊥ >ge = 2.0023 and A∥ >A⊥ which are the characteristic features of Cu2+ ions located in a distorted octahedron, elongated along the z-axis. Bonding parameter values revealed that the in plane s-bonding as well as in plane π-bonding are moderately ionic, and while out of plane π-bonding are to be ionic.