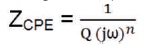
where w is the angular frequency (ω = 2πf), where f is the frequency in Hz, j2 = -1, 'Q' and 'n' are frequency independent fit parameters. The value of n is 0 ≤ n ≤ 1. When n = 1, then CPE is equivalent to pure capacitor (Q = C) and when n = 0 then CPE represents a pure resistor (Ningshen et al., 2011).
2.3.2 Potentiodynamic Polarization Measurement
Potentiodynamic polarization tests were conducted to investigate the corrosion mechanism. Corrosion parameters, such as anodic tafel slope (βa), cathodic tafel slope (βc), corrosion potential (ECorr), and corrosion current density (ICorr) were obtained from the potentiodynamic curves by using tafel extrapolation technique. The potentiodynamic polarization curves were initiated at -250 mV to +250 mV relative to open circuit potential. The experiments were carried out at the scan rate 5 mV/s and polarization experiment was continuous till the breakdown of transpassive potential occurred. All electrode potential were considered against reference electrode (SCE). The corrosion rate (CR) was calculated by using the Equation,
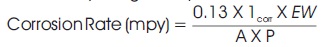
2.4 Characterization of Surface
The phase structure was investigated using Rigaku X-ray diffractometer, with Cobalt target (λ = 0.179 nm) and iron filter operating at 30 mA and under a voltage of 40 kV. The specimens were scanned over 2θ range of 20o - 50o with scanning speed 2o /min record the intensities. The Scherrer's equation was used to calculate the crystallite size from XRD data (Singh et al., 2013).
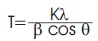
Where, T is the average crystallite size in nm, λ is the X-ray wavelength in nm, b is the line broadening in radian, θ is the Bragg angle in degree, and K is the constant that represent shape factor (K = 0.89 for spherical, 0.94 for cubic, and 0.9 for unknown size particles).
The surface morphology of the exposed samples was analysed using Scanning Electron Microscopy (SEM) (JEOL JSM-6610LV) with magnification range 40 KX. The micrographs were to identify the pits and micro-cracks after 24 hours immersion in different electrolytes.
3. Results and Discussion
3.1 Analysis of Stainless Steel 304
3.1.1 EIS Analysis
The Nyquist plots of 304 SS in Ringer solution, Wine, Beer, and 3.5% NaCl solution are shown in Figure 1.
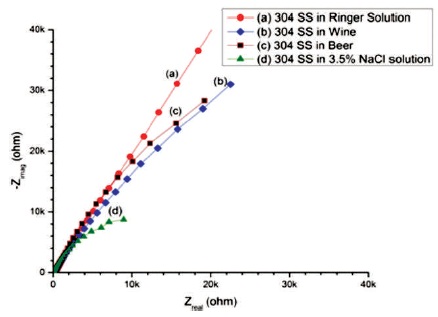
Figure 1. Nyquist Plots of 304 SS in (a) Ringer Solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
In the Figure, the semi-circle starts near the origin at high frequency region and shows a value of Rs at intersecting point on real axis. The other intersecting point on real axis away from the origin at low frequency region represents sum of Rs and Rp. The rise of semi-circle to Y-axis shows the capacitive behaviour. The result were interpreted using an equivalent circuit Rs(Rp ǀǀ CPE). All measured parameters are given in Table 1.
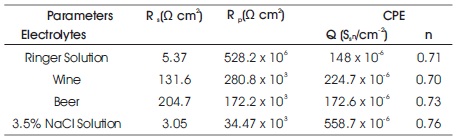
Table 1. Fitting Results of EIS Parameters for 304 SS
The results shows that polarization resistance (Rp = 528.2 x 106Ω cm2) of 304 SS immersed in Ringer solution is higher than 304 SS in other electrolytes and represented by larger the diameter of unfinished semi-circle as shown in Figure 1(a). The increase in the semi-circle arc indicated an increase in the film stability on the specimen and decrease in the semi-circle diameter indicates decrease in the passive film resistance (Ningshen et al., 2011).
The decreasing arc of 304 SS in Wine than 304 SS in Ringer solution shown in Figure 1(b), is characterized by a formation of weak protective passivating layer, which indicates less corrosion resistance. The unfinished semicircle of 304 SS in Beer with polarization resistance (Rp= 172.2 x 103Ωcm2) suggest that 304 SS in Beer is low corrosion resistance than Wine (Alar et al., 2016). The 3 2 polarization resistance (Rp = 34.47 x 103Ωcm2) of 304 SS in 3.5% NaCl solution is lower than the Rp of 304 SS immersed in the other three electrolytes, which is represented by small and depressed semi-circle as shown in Figure 1(d). The depressed semi-circle is either due to the presence of pores on the surface of working electrode. The order of corrosion resistant of SS 304 in different electrolytes is Ringer solution > Wine > Beer > 3.5% NaCl solution.
Bode plot represent the three parameters, which are needed to characterize the impedance. Those three parameters are magnitude 'ǀZǀ', phase angle ‘Ø ', and frequency 'f'. In Bode plot, curves have three frequency regions, such as low, high, and intermediate regions.
In Figures 2(a), (b), (c), and (d), bode spectra are presented for different electrolytes, such as Ringer solution, Wine, Beer, and 3.5% NaCl, respectively. These consists of two curves on one graph, solid lines represent the impedance modulus and dash lines represent the phase angle.
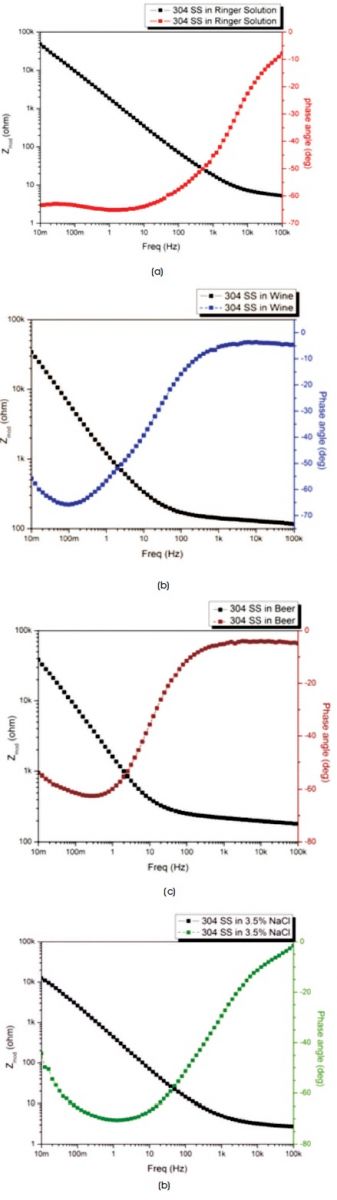
Figure 2. Bode Plots of 304 SS in (a) Ringer Solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
In all figures, the progress of impedance modulus vs. frequency reveals a area of stability at high frequency region and phase angle close to 0 , which shows that circuit behaves as a resistor and illustrate the values of Rs shown in Table 1. At intermediate frequency region, capacitive behaviour of the circuit exist, so impedance curves dependent on the frequency and phase angle approaches to -90o. At low frequency region, area stability of the impedance curve confirm the Rs + Rp value, which shows that the circuit behaves as a resistor and phase angle approaches to 0o, but this frequency independent region of curve is exterior from the plots.
3.1.2 Potentiodynamic Polarization Analysis
Figure 3 shows the potentiodynamic scan of 304 SS samples after 24 hours immersed in four different electrolytes, such as Ringer solution, Wine, Beer, and 3.5% NaCl solution.
All measured parameters of the potentiodynamic polarization measurement are given in Table 2. The results show that the corrosion current density of SS 304 specimen immersed in Ringer solution (ICorr = 0.20 μA and ECorr = -255 mV) is lower than SS 304 specimen immersed in other three electrolytes. So the polarization curve of 304 SS in Ringer solution is shifted towards left in comparison to other three electrolytes
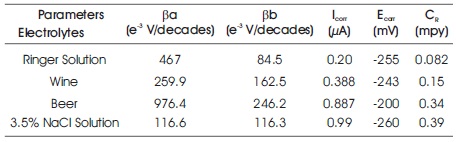
Table 2. Corrosion Parameters of 304 SS in (a) Ringer Solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
These shift comparison clearly is shown in Figure 3 by curve (a). The polarization curve (d) of 304 SS in 3.5% NaCl solution shifted toward right in comparison to others as shown in Figure 3 shows the lower affinity of corrosion than others.
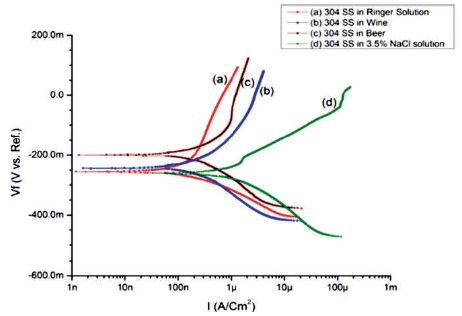
Figure 3. Potentiodynamic Curves of 304 SS Specimens in (a) Ringer Solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
The polarization curves (b) of 304 SS in Wine shifted toward right than curve (a) indicate that 304 SS in Wine is low corrosion resistant than in Ringer solution. And the polarization curve © shifted toward left of curve (d) signify that 304 SS in Beer is more corrosion resistant than 304 SS in 3.5% NaCl solution, but lower than 304 SS in Wine and Ringer solution.
In addition, SS 304 in 3.5% NaCl solutions exhibit a higher corrosion rate (0.39 mpy). While Ringer solution exhibit a lower corrosion rate of (0.082 mpy). It is found that 304 SS reveals to enhance the corrosion resistance in Ringer solution than the Wine, Beer, and 3.5% NaCl solution. The order of corrosion resistant of SS 304 in different electrolytes is Ringer solution > Wine > Beer > 3.5% NaCl solution.
3.1.3 Structural Analysis (XRD)
The XRD scan of 304 SS after immersing in Ringer solution for 24 hours to corrosion testing, indicate the crystallite structure as shown in Figure 4(a). According to Scherrer formula, crystallite size was 367.78 nm. Higher crystalline lead to longer life of stainless steel in Ringer solution (Singh et al., 2013) and signify the best corrosion resistant behaviour. While 304 SS remain amorphous after immersing in Wine, Beer and 3.5% NaCl solution and exhibits a broad with Full Width Half Maximum (FWHM) more than 5o as shown in Figure 4(b), (c), and (d), respectively.
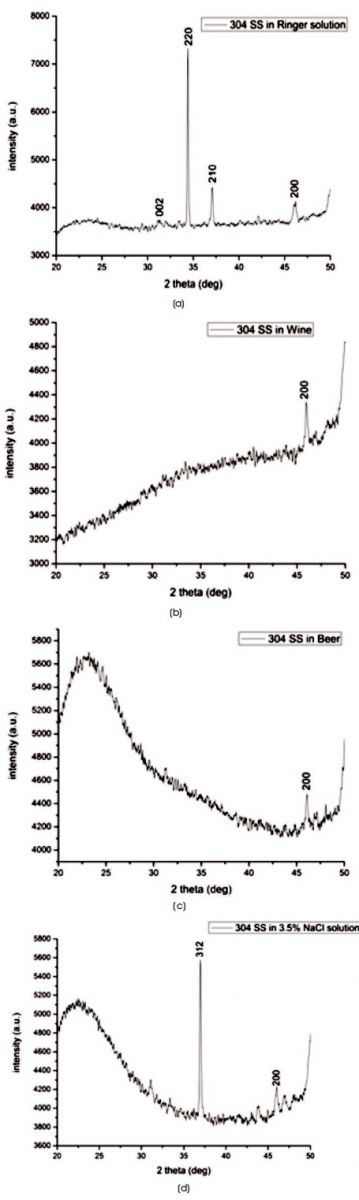
Figure 4. XRD Profile of 304 SS After Corrosion Testing in (a) Wine (b) Beer (c) 3.5% NaCl Solution
3.1.4 Morphological Analysis (SEM)
The surface morphology of 304 SS sample shows a tiny stain in Figure 5(a) during immersion in Ringer solution, which suggest that 304 SS has high corrosion resistant in Ringer solution. The small cracks on the surface of 304 SS were observed after immersion in Wine in Figure 5(b), where as the surface of 304 SS had formation of both cracks and pits after immersion in Beer in Figure 5(c) that advised the decline in corrosion resistance. The 304 SS subjected to simulated marine atmosphere has large volume of pits as shown in Figure 5(d) suggest for low corrosion resistant. The pit initiation due to dissolution of Manganese Sulphide (MnS) is present in 304 SS subjected to simulated marine atmosphere (Lv et al., 2015).

Figure 5. SEM Images of 304 SS samples After Corrosion Testing in (a) Ringer Solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
3.2 Analysis of Stainless Steel 316L
3.2.1 EIS Analysis
The Nyquist plots of 316L SS in (a) Ringer solution, (b) Wine, (c) Beer, and (d) 3.5% NaCl solution are as shown in Figure 6. All measured parameters are given in Table 3.
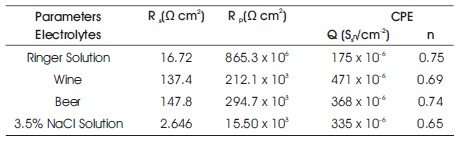
Table 3. Fitting Results of EIS Parameters for 316L SS
The results show that polarization resistance (Rp = 865.3 x 106 Ω cm2) of 316L SS immersed in Ringer solution is higher than 316L SS in other electrolytes and larger the diameter of unfinished semi-circle as shown in Figure 6(a), which mean 316L SS in Ringer solution is more corrosion resistant than all others. The decreasing arc of 316L SS in Beer than in Ringer solution indicates the less corrosion resistant than in Ringer solution. The semi-circle arc of 316L SS in Wine is shifted toward down as compared to 316L SS in Ringer solution and Beer as shown in Figure 6(b) signify the lower corrosion resistant behaviour of 316L SS in Wine than Beer and Ringer solution. The depressed semi-circle of 316L SS in 3.5% NaCl solution as shown in Figure 6(d) is observed by localized corrosion attack on the surface of the specimen. The order of corrosion resistant of SS 316L in different electrolytes was Ringer solution > Beer > Wine > 3.5% NaCl solution.
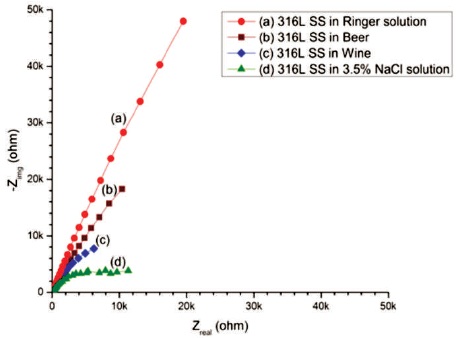
Figure 6. Nyquist Plots of 316L SS in (a) Ringer solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
The Bode spectra of 316L SS after immersed in four different electrolytes, such as (a) Ringer solution, (b) Wine, (c) Beer, and (d) 3.5% NaCl solution are presented in Figure 7.
In Figures 7(a), (b), (c), and (d), the progress of impedance modulus vs. frequency reveals that area of stability at high frequency region and phase angle approach to 0o, which shows circuit behaves as a resistor and illustrate the values of Rs which is shown in Table 3. At intermediate frequency region due to capacitive component phase shift act and phase angle approaches to -90o and impedance varies with the inverse of the frequency and reach to high impedance as shown in all figures. The lift of solid curve at low frequency region in Figure 7(a) suggests for high impedance of 316L SS in Ringer solution.
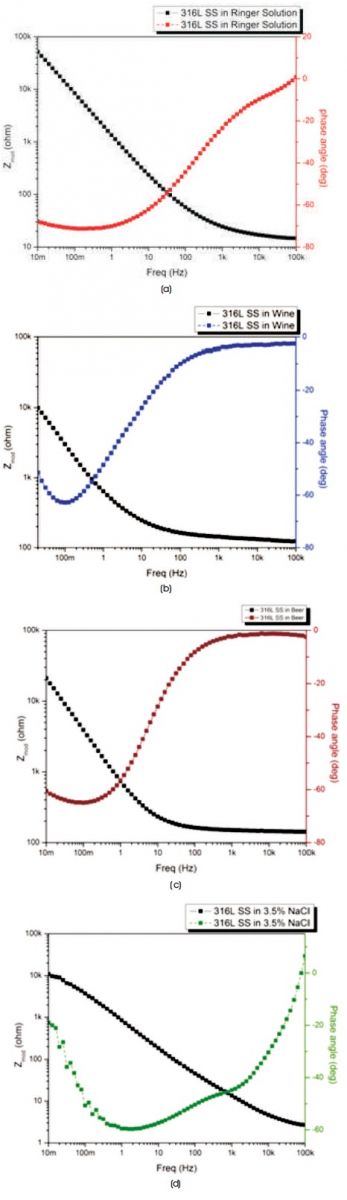
Figure 7. Bode Plots of 316L SS in (a) Ringer solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
3.2.2 Potentiodynamic Polarization Analysis
Figure 8 shows the potentiodynamic scan of 316L SS samples after 24 hours immersed in different electrolytes, such as Ringer solution, Wine, Beer, and 3.5% NaCl solution. The measured parameters of the potentiodynamic polarization measurement are given in Table 4.
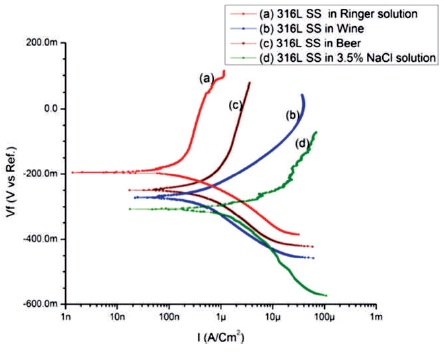
Figure 8. Potentiodynamic Curves of 316L SS Specimens in (a) Ringer solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
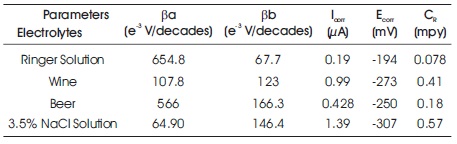
Table 4. Corrosion Parameters of 316L SS Specimens in (a) Ringer Solution (b) Wine (c) Beer (d) 3.5% NaCl Solution
The results show that the corrosion current density of SS 316L specimen immersed in Ringer solution (ICorr= 0.19 μA and ECorr = -194 mV) is lower than SS 316L specimen immersed in other three electrolytes. The polarization curve (a) of 316L SS in Ringer solution is shifted toward left in comparison to other three electrolytes as shown in Figure 8. The corrosion current density of SS 316L specimen immersed in 3.5% NaCl solution (ICorr = 1.39 μA and ECorr = - 307 mV) is higher than the SS 316L immersed in other three electrolytes with polarization curve (d) shifted toward right in comparison to others as shown in Figure 8, which shows the lower similarity of corrosion than others. The polarization curve (b) of 316L SS shifted toward left than curve (d) indicates the higher corrosion resistant in Wine than 3.5% NaCl solutions. And the polarization curve (c) shifted toward left than the curve (b) and (d) and toward right than curve (a) as shown in Figure 8, designate that in Beer, 316L SS is more corrosion resistant than in Wine and 3.5% NaCl solution but less than in Ringer solution.
In addition, 316L SS in Ringer solution exhibit a lower corrosion rate (0.078 mpy). While in 3.5% NaCl solution exhibit a higher corrosion rate (0.57 mpy). It is found that 316L SS disclose to improve the corrosion resistance in Ringer solution than the Beer, Wine, and 3.5% NaCl solution. The order of corrosion resistant of SS 316L in different electrolytes was Ringer solution > Beer > Wine > 3.5% NaCl solution.
3.2.3 Structural Analysis (XRD)
The XRD scan of 316L SS after immersing in Ringer solution and Beer for 24 hours for corrosion testing signifies the crystallite structure as shown in Figures 9(a) and (b) with average crystallite size as 1653.84 nm and 64.83 nm, respectively, which signify the higher corrosion resistance of 316L SS in Ringer solution. While 316L SS remain amorphous after immersing in Wine and 3.5% NaCl solution and exhibit a broad with FWHM more than 5o as shown in Figure 9(c) and (d).
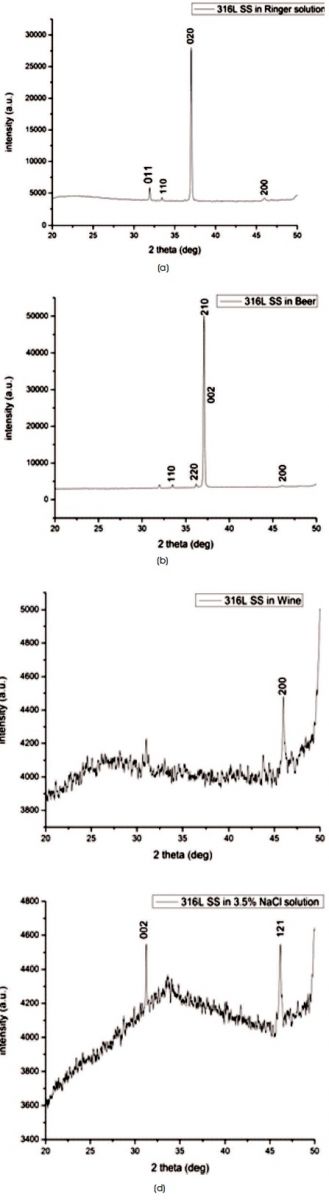
Figure 9. XRD Profile of 316L SS after Corrosion Testing in (a) Ringer Solution (b) Beer (c) Wine (d) 3.5% NaCl Solution
3.2.4 Morphological Analysis (SEM)
The SEM image of 316L SS sample after corrosion testing in Ringer solution shows the pits formation like stain on the surface as shown in Figure 10(a). The pits formation increase after corrosion testing in Beer and Wine as compared to Ringer solution as shown in Figures 10 (b) and (c), respectively. The large volume pits formed on 316L SS surface after corrosion testing in 3.5% NaCl solution as shown in Figures 10(d), which suggest for low corrosion resistance of 316L SS in 3.5% NaCl solution.
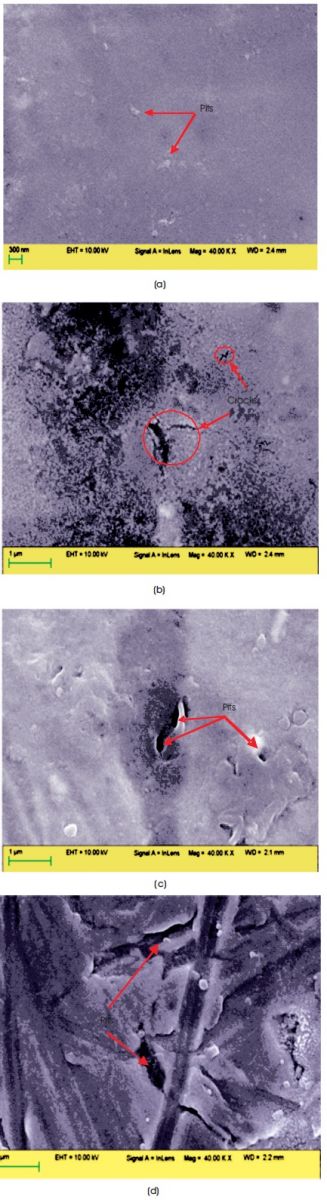
Figure 10. SEM Images of 316L SS Samples After Corrosion Testing in (a) Ringer Solution, (b) Wine (c) Beer (d) 3.5% NaCl Solution
Conclusions
The following conclusions have been drawn from present study,
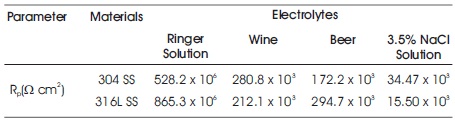
Table 5. Comparison Results of Corrosion Resistance for 304 SS and 316L SS
- In Ringer solution and Beer, 304 SS exhibit low corrosion resistance than 316L SS, While in Wine and 3.5% NaCl solution, 304 reveal the high corrosion resistance than 316L SS as shown in Table 5.
- According to Scherrer's formula, crystalline size of 316L SS is more than 304 SS after corrosion testing in Ringer solution. While both grade (304 SS and 316L SS) in Wine and 3.5% NaCl solution remain amorphous.
- A comparison of SEM micrograph of 304 SS and 316L SS in Ringer, Beer, Wine and 3.5% NaCl solution showed that 304 SS and 316L SS after corrosion testing in Ringer solution retain its morphology, hence making the surface less porous and more corrosion resistant, where as pits and cracks increase after exposure to corrosion testing in Wine, Beer, and 3.5% NaCl solution.