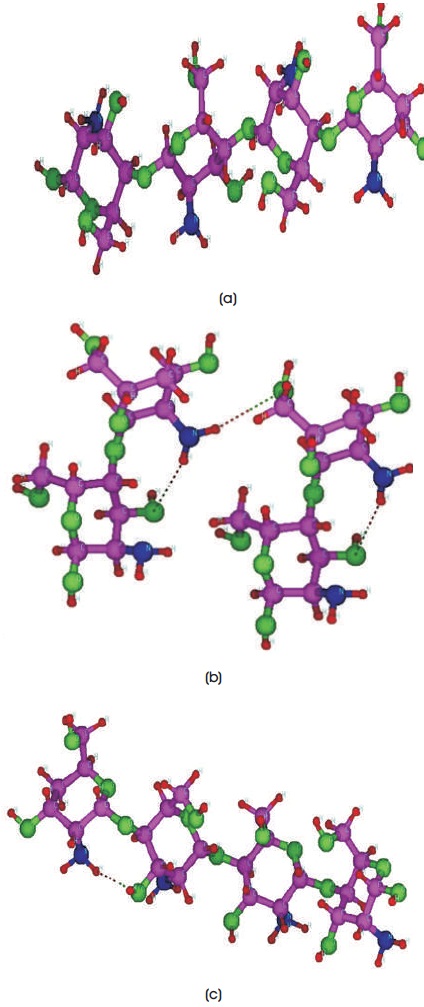
Figure 1. Molecular Structures of (a) Trans-chitosan, (b) 2D-chitosan, and (c) Cis-chitosan where C, N, O and H Atoms are shown as Purple, Blue, Green and Red Ball respectively
The structural, electronic and chemical bonding properties of biopolymer chitosan in tetramer structures are investigated by using density functional theory. These polymer structures exhibit structural activity relationship because the activity of these polymer isomers varies with the structural modification and molecular weight. In this approach the Generalized Gradient Approximation (GGA) is used for the exchange-correlation potential. In density of states analysis the calculated forbidden energy gap is reduced by 2.23eV for cis-chitosan structure, deduces its semi-conducting behaviour with decreased crystallinity and improved bioactivity. Hirshfeld population and charge density techniques are used to analyse electrostatic interactions and the charge distribution within these molecules. Crystal orbital overlap population shows ionic interactions and provide chemical bonding information. Modification of chitosan improves the properties and provide the better functionally improved bio-polymer. This study is aimed to be used in various chemical industries, pharmaceutical and biomedical fields.
Biopolymer chitosan is an attractive natural polysaccharide. The natural polysaccharides have their specific bioactivities and physicochemical properties, which depends on the structures and molecular weight. Molecular modifications alter the physicochemical properties and generate new functional bioactivities of polysaccharides. Biopolymer chitosan has been extensively studied for various applications such as medicinal, industrial, agricultural, papers and textiles, pharmaceutical, technical fields etc, because of its unique properties like flocculating, cost effective, biocompatibility, biodegradability, cosmetic, poly electrolyte, high mechanical strength, good adhesion, antibacterial function, hydrophilicity, degree of polymerization, chelating, and ion exchange properties etc (No & Meyers, 1989; Kawada, Yui, Okuyama, & Ogawa, 2001; Yang, Yang, Liu, Shen, & Yu, 2004; Kurita, 2006; Yin et al., 2008). Chitosan can be processed into various forms such as membranes, gels, films, bread, sponges, micro-particles, nano-fibers, and nanoparticles, therefore useful in variety of biomedical applications such as wound healing, gene therapy, tissue engineering, immunity promoter, antitumor agent, drug delivery, biomedicine etc (Dutta, Dutta, & Tripathi, 2004; An et al., 2011). In pharmaceutical field it is used as a carrier in controlled release drugs, as a filler in tablets and to mask the oral drugs which have bitter tastes in mouth. Chitosan has a positive ionic charge which shows the ability to attract the negative charge (Luo, Xu, Du, & Chen, 2004; Mourya & Inamdar, 2008; Skovstrup, Hansen, Skrydstrup, & Schiøtt, 2010). All these properties are based on different parameters such as processes used to condition it (dissolving, drying precipitation etc), its origin, molecular weight, molecular structure and its characteristics. Crystalline nature of polymer obstructs the ion movement and flexibility (Wu, Yang, Wang, Hu, & Fu, 2005; Tang, Qian, & Shi, 2007). Molecular modification can affect their mechanical and electronic properties. Some changes in the structures of polysaccharide deduce the different properties of material because molecular modification can change the position, type, numbers, dimensional structure, molecular weight and the substitution groups, which affects the bioactivities of polysaccharides (Okuyama et al., 2000; Oyebamiji & Semire, 2016).
Molecular modification methods include biological, physical and chemical changes; these methods are mainly used to observe the effect on bioactivities, morphology and conductivity of the polysaccharide. Biopolymer chitosan is a natural carbohydrate derived from deacetylation of chitin, a major component of the shells of crustacean such as crab, shrimp, and crawfish. Chitosan is a high molecular co-polymer, containing reactive amino and hydroxyl functional groups, it is the only natural positive ion polysaccharide therefore it is gaining extensive attention of researchers (Li et al., 2016; Sahariah & Masson, 2017; Gryczka, Gawrońska, Migdał, Gawroński, & Chmielewski, 2008). There are many research works have been done in pure chitosan and their derivatives but no theoretical calculations done in tetramer forms of chitosan till now. In this work the authors have presented a comparative study of three possible and different chemical structures (in tetramer forms) of chitosan. This study relates to the structural-activity relationship because of the structural-activity relationship (Martins et al., 2014; Goy, Britto, & Assis, 2009) based on chemical structures of a material and its biological activity. This is a very useful technique in the biomedical and pharmaceutical field. A little change in the chemical structure of biomedical compound, changes the bioactivity of material and provide information about drug potency. The experiment values exhibit that antibacterial activity and inhibition range for microbial growth of water soluble chitosan was higher than the water insoluble chitosan (Kamala, Sivaperumal, & Rajaram, 2013). The present work inspired by an experimental work (Kim, 2018), reported that the electronic properties plays very significant role in the inhibition mechanism of microorganism. Positively charged chitosan can easily interact with negatively charged lipids, carbohydrates and proteins, which are located on the cell surface of bacteria, exhibits inhibition in the growth of bacteria. Therefore, the authors are reporting electronic properties, chemical bonding properties and charge distribution of chitosan to check out its bioactivities.
In this paper chitosan tetramer structures (i) trans-chitosan, (ii) 2D-chitosan and (iii) cis-chitosan are studied by using density functional theory within the SIESTA computational program. To enhance its solubility and physicochemical properties, it is needed to modify the pure structure of chitosan. Hydrogen bond and molecular weight (MW) play very crucial role in bioactivities and morphology of polymer matrix in the structural-activity relationship study (Ugwu, Ezema, Eze, & Ugwuja, 2014). Solubility of biopolymer chitosan enhances its application specially as an antibacterial agent but on the other hand restricts the biological application due to its insolubility and low solubility (Goy, Morais, & Assis, 2016). Poor solubility of chitosan is caused by high crystalline behaviour of polymer matrix because the behaviour of chitosan polymer is affected by the number of hydrogen bonds (Chang, Wu, & Tsai, 2018). The presence of positive ionic charge gives it to chemically bind with negative charge like fats, lipids, bile acid etc (Jiao, Yu, Zhang, & Ewart, 2011; Badawy & Rabea, 2011; Ramkumar & Sundaram, 2016a; 2016b). It is already reported that (Chien, Sheu, Huang, & Su, 2007) the presence of free amino group in chitosan shows higher antibacterial effect.
In this work, trans-chitosan shows high crystalline nature and hydrophobic property due to lack of hydrogen bonds, whereas 2D-chitosan and cis-chitosan structures have hydrogen bonds which shows hydrophilic property, ion concentration in the amorphous region and increase of bioactivity.
All the parameters of calculations of the structural, electronic and chemical bonding properties of chitosan are performed in the framework of density functional theory (DFT) with SIESTA package in order to gain deeper analysis into the behaviour of these compounds. DFT calculations provide precise descriptions of the structure and a good balance between the computational cost and accuracy of the results, which inspired the authors to study these structures in detail. Avogadro software is used to create tetramer structures of chitosan (C6H13O5N)n, n=4, where two more hydrogen atoms are used to create the 2D-chitosan structure (Hanwell et al., 2012). The calculated molecular weight (MW) for cis and trans structures are 646.64, 646.64 grams/mole respectively and for 2D chitosan structure 648.66 grams/mole. Theoretical modelling can provide accurate prediction of structures and their characterization. The normconserving pseudo-potentials are built within the Generalized Gradient Approximation (GGA) method following Perdew-Burke-Ernzerhof (PBE) and a double-zeta polarized basis set is used (Perdew & Zunger, 1981; Wen et al., 2012). Many approximations to the exchange correlation energy are developed and implemented, but the authors consider PBE-GGA because they found that the PBE functional show accuracy in ionization potential, electron affinity, bond length and many other results. The equilibrium geometries of chitosan structures are analysed for three different configurations shown in Figure 1(a-c), (a) trans-chitosan (b) 2D-chitosan (c) cis-chitosan. The optimization process of these structures is done with an equivalent real space mesh cut-off 250 Ry and 1x1x9 Monkhorst-Pack grid. For structural properties, energy(E)- volume(V) values, for electronic structure properties, the density of states (DOS) and projected density of states (PDOS), for atomic charge analysis Hirshfeld population, charge density techniques and for the chemical bonding properties crystal orbital overlap population technique are used to calculate the results.

Figure 1. Molecular Structures of (a) Trans-chitosan, (b) 2D-chitosan, and (c) Cis-chitosan where C, N, O and H Atoms are shown as Purple, Blue, Green and Red Ball respectively
Structural properties of these fully relaxed configurations are assessed from the energy and volume values, which are represented by E= f(V) curves shown in Figure 2(a-c). The calculated total energy and cell volume values for Figure 2(a) -12486.17eV, 804.9Å3, (b) -12523.33eV, 1067.99Å3 and (c) -12489.44eV, 880.0Å3 respectively. The molecule with larger volume allows larger ion transport and drug binding property. Larger total energy and less volume value of trans-chitosan structure indicates the higher crystallinity than 2D and cis-chitosan structures. Higher crystallinity restricts the ion movement therefore it can be observed that the unavailability of hydrogen bond in trans-chitosan exhibits hydrophobic property and nonpolar covalent bonding character of molecules. This structure does not provide enough space to the ion movement which restricts the flexibility of polymer matrix. The hydroxyl and amine groups in the chitosan structures are responsible for their activities and the attractive biological properties are strongly dependent on water solubility, hence the hydroxyl and amine groups are found less reactive in trans-chitosan structure, which causes insolubility in water. The biological activities of transchitosan are hard to investigate because of the reasons stated earlier. The 2D and cis structure of chitosan consist of larger volume than trans-chitosan.
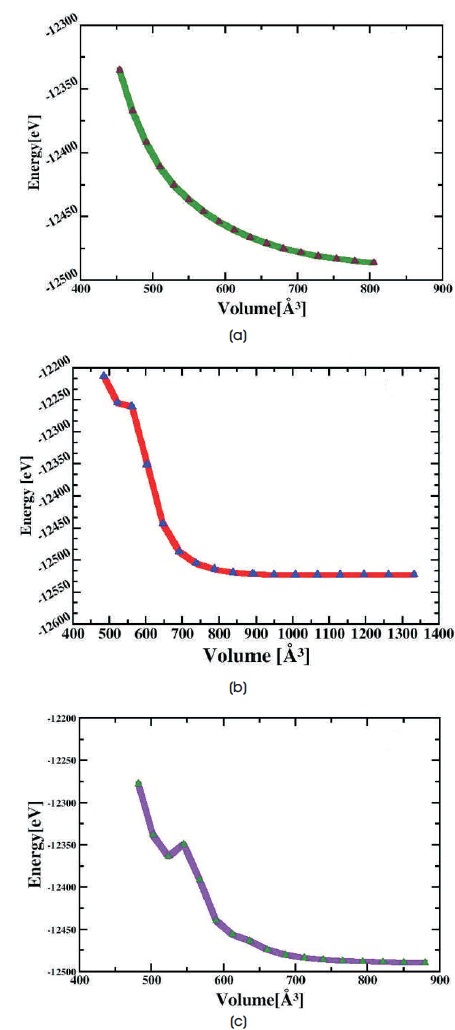
Figure 2. Energy-Volume Curves of 2(a) trans-chitosan, (b) 2D-chitosan and (c) cis-chitosan
Density is inversely proportional to the volume of a material therefore trans-chitosan is denser than 2D and cis-chitosan. It is well-known that the solid state of a substance occupies less volume than the liquid state for same substance but a notable exception is water. Water is less dense/larger volume in its solid form than its liquid form because of the perfectly ordered hydrogen bonding. When a lot of hydrogen bonds are added together, they can have a significant influence on the structure of molecules, therefore ice floats in the water. In the experimental work, (Chang et al., 2018) it is observed that the larger molecular weight of chitosan has denser structure, it results stronger intra-molecular hydrogen bonds and restrictive hydroxyl and amine group, whereas in the 2D-chitosan larger molecular weight indicates dense structure but least total energy and larger volume values exhibit less dense and semi crystalline morphology. Therefore 2D chitosan geometry indicates moderate strength of intra-molecular hydrogen bonds, low solubility and gel like property. It can be clearly seen in its molecular structure that the two polymer chains are physically cross linked by hydrogen bonds, exhibits the self healing hydrogel property and high swelling ability. The combined form of hydrophobic effects and hydrophilic effects in the hydrogel structure causes high swelling ability (Metzler, Chylińska, & Kaczmarek, 2015; Tyliszczak, Drabczyk, Kudłacik-Kramarczyk, Bialik-Wąs, & Sobczak- Kupiec, 2017). In cis-chitosan structure availability of hydrogen bond shows fully hydrophilic property and polar covalent bonding character of molecules therefore it provides proper space to the ion movement and shows larger amorphicity than 2D chitosan (Franca, Lins, Freitas, & Straatsma, 2008; Soler et al., 2002). Molecular volume directly connected to the transport and binding of a drug molecule to exert its therapeutic potency. The less dense structure of cis-chitosan indicates that the effect of intramolecular hydrogen bond is weak and more ion transport possibilities than the other two structures. The ion transport and interactions would be more clarify by the Density of States (DOS), Projected Density of States (PDOS), hirshfeld population, charge density analysis and COOP technique. To understand the morphology, conductivity and bioactivity precisely, DOS/PDOS analysis of these polymer matrices are correlating to the volume-energy, partial charges distribution and chemical bonding results. These results can provide a clear vision to the possible physicochemical properties.
DOS and PDOS curves play very important role to calculate the forbidden energy gap (Eg) between the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO), it is termed as HOMO-LUMO gap, which relates to the charge transport and conductivity. HOMO energy shows the ability of an atom to donate the electrons to the receptor and LUMO energy shows the ability to accept the electrons from the receptor (Selvaraju, Jothi, & Kumaradhas 2013; Rangel, Rignanese, & Olevano, 2015).
Reduced energy band gap shows better ability of an atom to donate the electrons to the neighbouring atom (Ravindran & Asokamani, 1997). Figure 3(a-d) shows DOS and PDOS of trans, 2D and cis structures of chitosan. In Figure 3(a) the calculated HOMO-LUMO gap of these structures are 3.7 eV, 3.0 eV, and 2.23 eV respectively, it exhibits electronically insulating behaviour of transchitosan and semiconducting behaviour of 2D-chitosan and cis-chitosan. In Figure 3(b-d) observed maximum contribution of nitrogen (N) atom in the valence band and hydrogen (H) atom in conduction band for density of states (DOS) and in PDOS of s-orbital maximum contribution of H atom is clearly seen in valence band and conduction band of all the structures. In PDOS of porbital maximum contribution of N2p is seen in valence band for all these structures but in the conduction band higher contribution made by C2p, O2p, and C2p in transchitosan, 2D-chitosan and cis-chitosan respectively. In Figure 3(d) contribution of O2p is not seen in the PDOS of porbital, shows weaker hybridization and empty 2pz orbital is an excellent candidate to accept the electron. There is decrease in crystalline nature and increase in amorphous nature, it causes increase in ion concentration, ion mobility and ionic conductivity of cis-chitosan structure (Djurovich, Mayo, Forrest, & Thompson, 2009; He, Pandey, Boustani, & Karna, 2010).
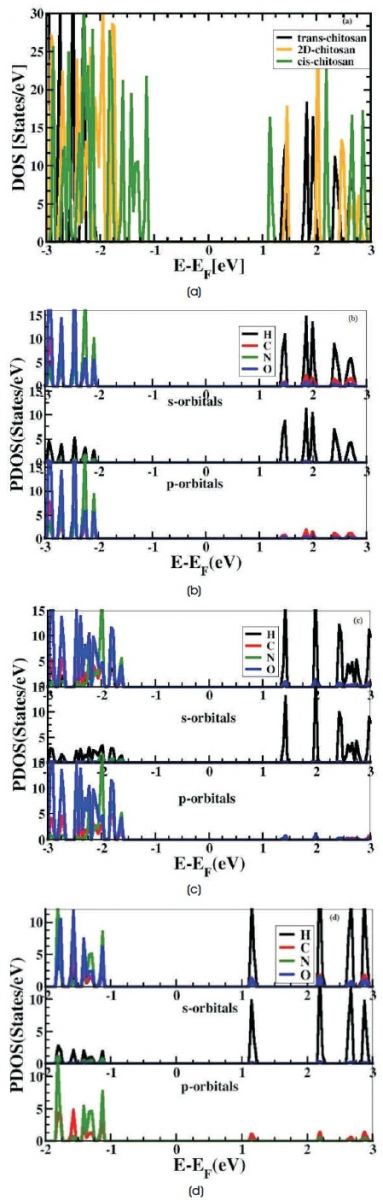
Figure 3(a-d). DOS and PDOS of Trans, 2D and cis Structures of Chitosan
From Frontier molecular orbitals (HOMO and LUMO) of chitosan, forbidden energy gap (Eg), ionization potential (I), electron affinity (A) and hardness (η) are calculated, shown in Table 1. The formula used for global hardness = (I-A)/2 where I= -EHOMO, A= -ELUMO (Akman, 2017). It is clearly seen that the least value of ionization energy observed for cis-chitosan. The least value of ionization energy 1.1eV indicates less energy required to remove the electron in cis-chitosan. Trans-chitosan with high ionization energy indicates that electrons are more tightly bound and difficult to remove. Trans-chitosan has harder matrix than the other two. A large energy band gap exhibits a high chemical stability therefore hard molecules have large forbidden energy gap. Hard molecule required large excitation energy from ground to excited state, whereas soft molecules have small band gap that means small excitation energy needed for an electron to excite from ground state. Thus, more charge transfer possible in soft molecules because they easily accept the changes in their electron number and distribution but hard molecules resist the changes. And hence soft molecules would be more polarizable and more reactive than hard molecules (Pearson, 1986). The calculated hardness value is slightly decreasing in the order of trans-chitosan>2Dchitosan> cis-chitosan. The Eg value 3.0eV and global hardness = 1.5eV confirms the semiconducting behaviour and gel like properties of 2D chitosan and indicate that moderate ion transport is possible. Therefore, 2D chitosan exhibits possibilities of some other type of bioactivities. The cischitosan with the Eg value 2.23eV and global hardness = 1.1eV confirms semiconducting behaviour and more flexible polymer matrix with higher bioactivities.
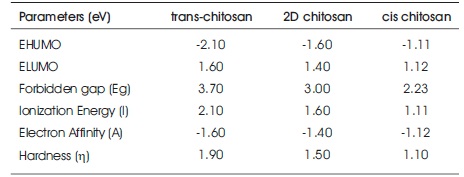
Table 1. Parameters Calculated by DFT-PBE Method of Tetramer Chitosan Structures
From Table 2 C-C,C-O,C-N bond lengths for trans-chitosan structure are 1.53 Å, 1.40 Å, 1.46 Å, for 2Dchitosan structure are 1.55 Å, 1.42 Å, 1.46 Å and for cischitosan structure are 1.57 Å, 1.43 Å, 1.47 Å. The observed bond lengths of trans-chitosan structure are smaller than the 2D and cis structures. Bond length decreases in the order of cis-chitosan >2D- stability of tetramer forms of chitosan also confirmed by cohesive energy. The calculated cohesive energy values of trans-chitosan, 2D chitosan and cis-chitosan are 5.43eV, 5.2eV and 5.01eV respectively by the formulachitosan>trans-chitosan, it also confirms the hardness of trans-chitosan polymer matrix. In cis-chitosan bonds are getting stretched, exhibits soft morphology due to their highly reactive amino and hydroxyl groups. All the results confirms that higher bioactivity is possible in the soft polymer matrix. The increasing order of bioactivity is trans-chitosan < 2D-chitosan < cis-chitosan. The structural stability of tetramer forms of chitosan also confirmed by cohesive energy. The calculated cohesive energy values of trans-chitosan, 2D chitosan and cis-chitosan are 5.43eV, 5.2eV and 5.01eV respectively by the formula

Where the term E total crystal is used for the total energy of the crystal, E isolated atom is for total energy of per atom and N is used for the number of total atoms in unit cell. Cohesive energy results that trans-chitosan is most stable structure and the stability of tetramer forms of the chitosan is reducing and ductility increasing with the structural modification.
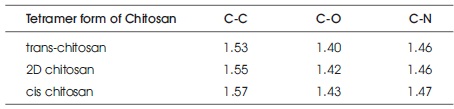
Table 2. Average bond length (Å) of tetramer forms of chitosan
Hirshfeld population shows atomic charge analysis, provide the information about molecular polarizability atomic charge effect, electrostatic interactions, reactivity etc. This population analysis calculate original and accurate partial charges (Hirshfeld, 1977). The hirshfeld population calculate their atomic partial charges on the basis of electron charge density at each point which is shared between neighbouring atoms.
In hirshfeld population the partial atomic charges are used as descriptors to describe the charge distributions in chemical binding theory. Hirshfeld population is found very reliable technique for the description of the charge distributions within the molecule. Partial atomic charges provide charge transfer information within the insulators and semiconductors surfaces, which indicates to their structural-activity relationship. The partial charges play very important role to describe the bioactivities of biomaterials in the theoretical modelling (Matczak, 2016; Abdallah, Gadzhiev, & Adnan, 2009; Bultinck, Van Alsenoy, Ayers, & Carbó-Dorca, 2007). In Table 3 C, H atoms have positive partial charges whereas O, N atoms have negative partial charges but some of the C atoms also consist the negative partial charge because they are influenced by the high electro-negativity of N and O atoms. It is observed that the highest electro negativity of N and O atom are (-0.217e), (-0.189e) for cis chitosan, (- 0.212e), (-0.189e) for 2D-chitosan and (-0.202e), (-0.184e) or trans-chitosan. Cis-chitosan shows higher polarizability, more reactive amino group and better electrostatic interactions (Marenich, Jerome, Cramer, & Truhlar, 2012; Wang, Li, & Truhlar, 2014) than 2D and trans structures because the more electronegative atom obtains a partial negative charge but the less electronegative atom obtains partially positive therefore nature of bonds become polar and their charge distribution is polarized (Tan, Guo, Lv, & Zhang, 2015). These results confirm high water solubility behaviour and antibacterial activity of cischitosan structure. For the better understanding of the inhibition mechanism the authors have studied the charge density of these structures shown in Figures 4-6.
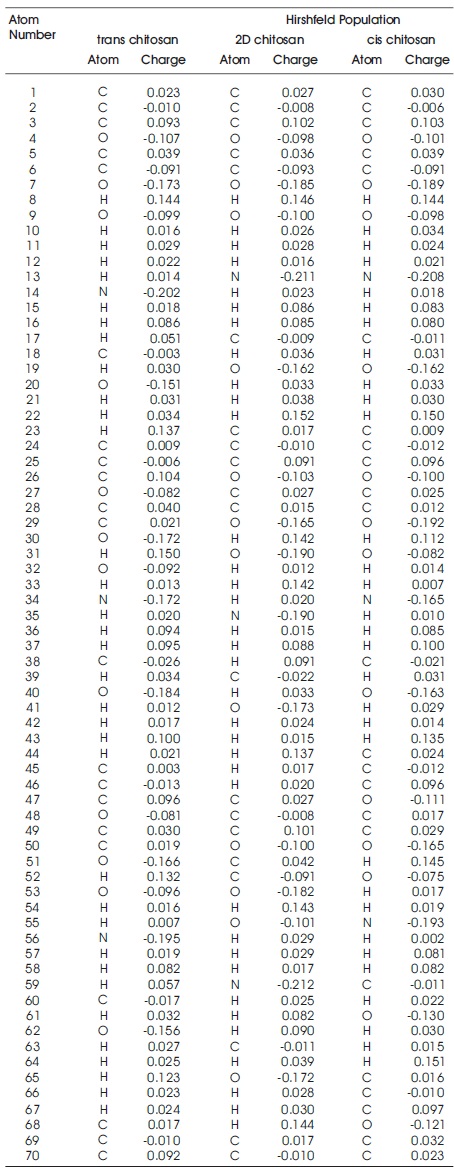
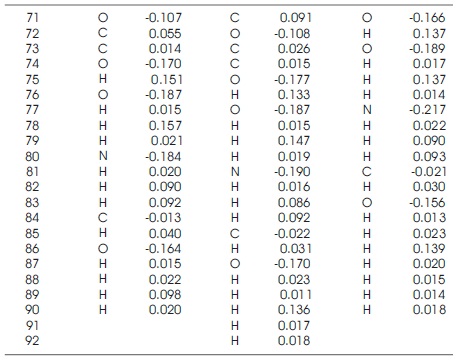
Table 3. Hirshfeld Population of Trans, 2D and Cis Chitosan Structures
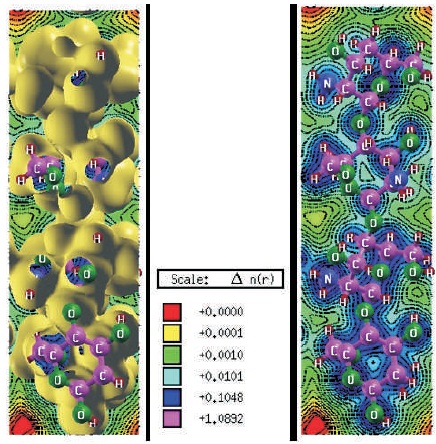
Figure 4. Charge-Density Curves of Trans-chitosan (in electrons/bohr3) (a) With Isosurface Value 0.01 (b) Without Isosurface Value
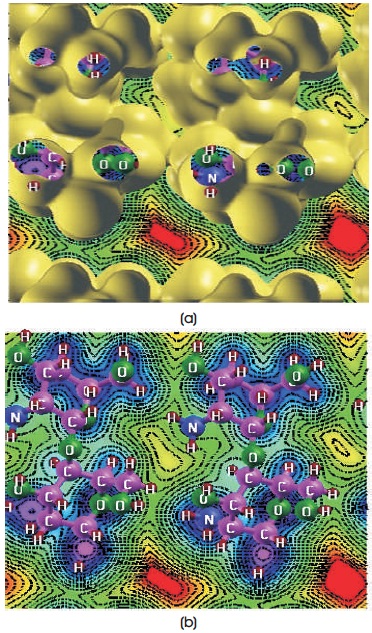
Figure 5. Charge-Density Curves of 2D-chitosan (in electrons/bohr3) (a) With Isosurface Value 0.01 (b) Without Isosurface Value
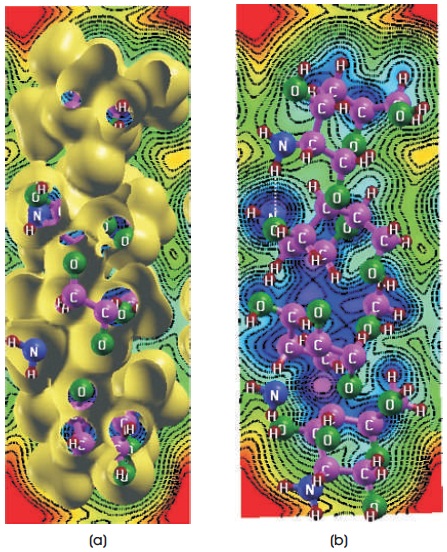
Figure 6. Charge-Density Curves of Cis-chitosan (in electrons/ bohr3) of (a) With Isosurface Value 0.01 (b) Without Isosurface Value
Figures 4a, 5a, 6a show electron-charge density change for trans, 2D and cis-chitosan structures with the 0.01 isosurface value and Figures 4b, 5b, 6b without isosurface value respectively. These charge density curves indicate that charge transfer (Amorim et al., 2013; Shafiee, Salleh, & Yahaya, 2011) and electrostatic interactions among the atoms, which are responsible for the biological activity. Molecular electrostatic potential scale describes the potential increases in the order of red< yellow< green
In cis-chitosan amino and hydroxyl groups are found more reactive. H+ ion concentration and the high density of positive charge is demonstrated on the structure. H+ ion shows greater electrostatic attraction to the negative electron cloud of the anions and have a greater distortion, therefore they have greater polarizing power. One free amino group is clearly seen on the surface which shows crucial role in the antimicrobial activity of chitosan. The antimicrobial activity comes from the cationic nature, ionic strength and low molecular weight of the cis-chitosan. These factors are decisive factors on the antibacterial activity because cis-chitosan can easily penetrate into the cell wall of bacteria and inhibit the growth of the bacteria. Whereas with the increase of molecular weight, the penetration capacity into cell wall decreases. The antibacterial activity of low molecular weight chitosan is higher than that of the high molecular weight chitosan. Therefore, 2D chitosan shows less antibacterial activity because of greater molecular weight and semi-crystalline morphology but can exhibit other activities such as antioxidant, self-healing hydrogel etc. (Goy et al., 2009; Liu et al., 2006). It is concluded that the polymers which show higher charge densities exhibited the improved antimicrobial activity.
The Crystal Orbital Overlap Population (COOP) diagrams are equivalent to a multiplication of the DOS spectrum and this technique is providing a comparative and explicit analysis of positive, negative, zero COOP intensities as bonding, anti-bonding and nonbonding atomic interactions respectively (Demazeau, Matar, & Poettgen, 2007; Matar, Pöttgen, Alam, & Ouaini, 2012). The chemical bonding interactions such as C-H, N-H ,C-C, C-O and C-N are described within overlap matrix shown in Figure 7(a-c). In Figure 7(a) C-C interaction is found in bonding site and C-O interaction in anti-bonding site with larger intensity but in non-bonding site there is no interaction is found. It deduces strong hybridization, high crystalline nature and harder matrix. In trans-chitosan molecule the -OH and -NH groups are less reactive and 2 difficult to dissociate, thus ion movements become difficult.
In Figure 7(b-c) C-C bond shows bonding interaction, C-O bond anti-bonding interaction with larger intensity and N-H bond shows non-bonding interaction, resulting weak hybridization (Hughbanks & Hoffmann, 1983; Matar & Kfoury, 2016) and H atom breaking bond with N atom. The increase in H+ ion concentration and mobility causes amorphous nature. According to the hard soft acid base theory hard acid H+ ion getting attached to the highly electro-negative hard base O- ion. The theory elaborates that the hard acids prefer to bond with hard bases and show more ionic character in its bonding (Artacho et al., 2011; Pearson, 1963; Gray, 1997).
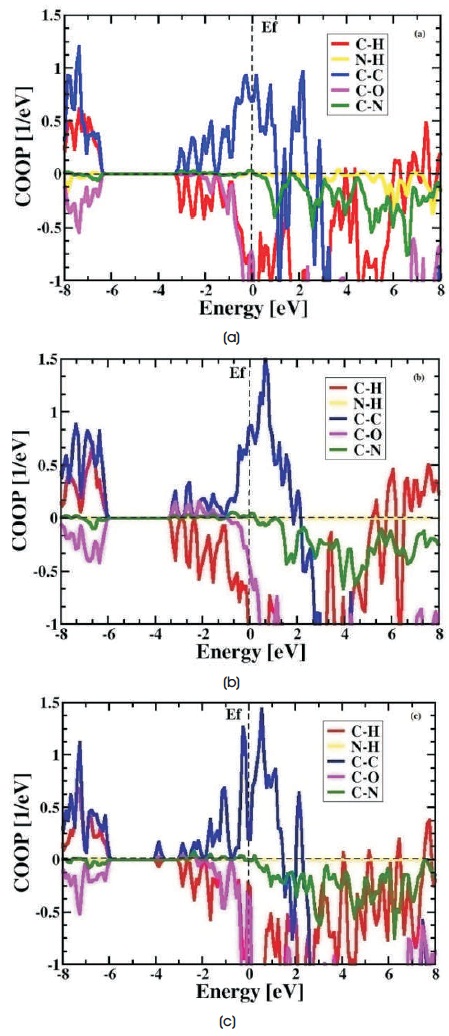
Figure 7. COOP Analysis of (a) trans-chitosan (b) 2D-chitosan and (c) cis-chitosan
The authors observed that the modification of these structures affects their potential for applications. The water insolubility of trans-chitosan structure is disadvantageous for its broad application as an antibacterial agent.
It is already discussed that in combination of the hydrophobic and hydrophilic interactions, result in the polymer matrix to swell and uptake water to form the hydrogel. These hydrogels are containing hydrophilic monomers by dangling side chain and they have ability to absorb water through hydrogen bonding, thus 2D-chitosan structure behaves as self healing hydrogel. This chitosan based hydrogel can be applicable in controlled drug delivery, self healing plastics and various medical field (Ostrowska-Czubenko, Gierszewska, & Pieróg, 2015). On the other hand cis-chitosan has only one hydrogen bond which decreases the hydrogen bond intensity therefore cis-chitosan shows good solubility in aqueous solution and can present excellent antibacterial activity by the chemically bind with anionic phospholipids such as E. coli, Salmonella Typhi, and other gram negative bacteria (Hafdani & Sadeghinia, 2011). Therefore water soluble chitosan compound is very useful to treat the diseases such as the urinary tract infection, typhoid, pneumonias etc. and to prevent the risk of side effect of synthetic drugs. In this study HOMO-LUMO gap is used to prove the bioactivity from charge transport within the compound. When the authors correlate all the Energy- Volume, DOS, PDOS, hirshfeld population, charge density analysis and COOP results, noticed that reduced HOMO-LUMO gap proves high chemical reactivity and more charge transport occurs in the cis-chitosan structure.
In this paper the structural-activity relationship study of three tetramer structures (trans,2D,cis) of chitosan is presented with in the density functional theory. Role of reduced HOMO-LUMO band gap and availability of hydrogen bond is clearly seen among these structures with different physicochemical properties. Cis-chitosan shows lower forbidden gap and lower energy band gap exhibits easy excitation of electrons, which enhances non-bonding interaction such as hydrogen bonding and hydrophilic interaction. It results in increase of hydrogen + ion (H ) concentration, ionic conductivity and its bioactivity as an antibacterial agent which is good agreement with the experimental work. The 2D, a physically cross linked structure of chitosan shows self healing hydrogel properties, high sorption and high swelling ability. The insolubility of trans-chitosan restricts the ion movement, ionic conductivity, its biological activity and application. From the obtained results it is clearly seen that the higher antibacterial activity of water soluble cis-chitosan and self healing hydrogel properties of 2D chitosan could be used in pharmaceutical, chemical and biomedical field. This study initiated a novel subject for both the experimental lists and theoreticians.
We gratefully acknowledge the kind support of the management of Shri Shankarachar ya Group of Institutions (SSTC). Helpful discussions with Neelam Srivastava (Banaras Hindu University), Prof. Ravindra Pandey (Michigan Technological University, USA) and Dr. Rodrigo Garcia Amorim (Universidade Federal Fluminense-UFF, Brazil) are acknowledged.