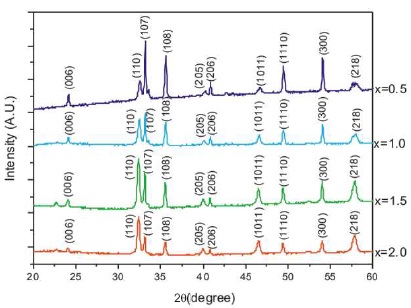
Figure 1. XRD Pattern of CaLaxFe12-xO19
La substituted M type Calcium hexaferrite with composition CaLaxFe12-xO19 (x=0.5, 1.0, 1.5, 2.0) were synthesized by sol gel auto combustion method. The prepared samples were characterized by XRD, SEM, EDAX, FTIR, and VSM. X ray diffraction study shows that all the species have hexagonal crystal structure with lattice constants of a =5.87 - 5.91 Å and c = 22.89 - 23.21 Å. There was increase in lattice volume with increasing La ion concentration. SEM images reveal that particles were hexagonal platelet-like shape, and the grain size increases with increase of La ion concentration. EDS measurements revealed the stoichiometric cationic ratios of the prepared samples. The absorption band between 520 cm-1 and 440 cm-1 in FTIR confirm the formation of hexaferrite. The magnetic properties of the samples were studied by VSM. The change in magnetic parameter results make substituted hexaferrite material suitable for recording media.
M-type hexaferrites possesses hexagonal crystal structure having good thermal durability, perfect chemical stability, unique magnetic and electrical properties, and corrosion resistivity is extensively used in recording devices, telecommunication, magneto-optical, microwave absorbers, military applications, and microwave devices (Auwal, Ünal, Baykal, Kurtan, & Yıldız, 2017). Keeping this view in mind, M type La doped Ca hexaferrite were prepared by sol gel auto combustion method. The unit cell of M type of hexaferrite is composed of the stacking sequence SRS*R*, where S* and R* blocks O are rotation of S and R blocks at 180 about the hexagonal c-axis. Within S block, there are three interstitial sites, one octahedral 2a site occupied by spin-up Fe3+ ion and two tetrahedral 4f1 sites occupied by spin-down Fe ions. Within R block, there are three interstitial sites, two octahedral 4f2 sites occupied by spin-up Fe3+ ions and one trigonal bi-pyramidal 2b site occupied by spin-up Fe3+ ion. There are three interstitial octahedral (12k) sites within each R-S interface layer, which are occupied by spin-up Fe ions (Mahmood, Awadallah, Bsoul, & Maswadeh, 2017).
The general formula of M type hexaferrite is MFe12O19, where M is usually barium, strontium, calcium, or Lead (Mama tha, Krishnaiah, Prakash, Rewatka r, & Nagabhushana, 2014). Calcium is richer than strontium and barium on the earth, and its price is relatively cheaper. As compared to barium and strontium hexaferrite, calcium hexaferrites have been less studied and has magnetic properties comparable to barium and strontium hexaferrite (Rewatkar, Prakash, & Kulkarni, 1996).
Kaur, Narang, and Bahel (2017) have synthesized the La substituted strontium hexaferrite SrLaxFe12-xO9 (x= 0, 0.10, 0.15, 0.20) by using citrate auto-combustion method. They observed that the SEM grain diameter decreases with La substitution from x=0.0 to x=0.10. Thereafter the grain diameter increases from x=0.10 to x=0.25. The saturation magnetisation and remanent magnetisation decrease with increasing La concentration while the coercivity increases from x=0.0 to x=0.10 thereafter the coercivity decreases with increasing La ion concentration (Kaur et al., 2017).
Azim, Atiq, Riaz, and Naseem (2014) synthesized the La substituted strontium hexaferrite SrLaxFe12-xO19 (x= 0, 0.10, 0.15, 0.20, and 0.25) by using citrate auto-combustion method. They observed that the crystallite size increases with increasing La concentration. The saturation magnetisation and remanent magnetisation decrease with increasing La concentration while the coercivity increases from x=0.0 to x=0.10 thereafter the coercivity decreases with increasing La ion concentration (Azim et al., 2014).
Singh et al. (2016) synthesized the La substituted barium hexaferrite BaLaxFe12-xO19 (x= 0.05, 0.10, 0.15, 0.20, and 0.25) by using citrate auto-combustion method. They have observed that the crystallite size decreases up to x=0.15 thereafter it increases. The saturation magnetisation and remanent magnetisation remains almost constant while the coercivity decreases with increasing La ion concentration (Singh et al., 2016).
The aim of the present work is to synthesize the high purity La3+ substituted Ca hexaferrite and to improve its magnetic properties to make these materials suitable for recording applications.
The samples of M-type La substituted hexaferrite with formula CaLaxFe12-xO19 (x=0.5, 1.0. 1.5, 2.0) were synthesized by sol gel auto-combustion method.
Stoichiometric amount of A.R. grade Calcium nitrate Ca(NO3)2 4H2O, Iron nitrate Fe(NO3)3 9H2O, Lanthanum nitrate La (NO3)3 9H2O, and Citric acid were dissolved in double filtered distilled water. The ratio of citric acid to metal nitrate ions was 1:1. The solution was neutralized to pH 7 by adding Liquor ammonia. The neutralized solution was heated at 80 oC on a hot plate with continuous stirring. After evaporation of water the solution became a viscous brown gel. As the temperature was increased further, dried gel is burnt in a self propagation combustion manner to form a loose powder. The powder was grinded in a pestle and mortar to form fine particles. The fine particles thus obtained were calcinated at 950 oC in an electric furnace for 4 hours to obtain ferrite nano particles.
The structural characterizations of the samples were performed by P analytical X' Pert Pro diffractometer with Cu-Kα radiation of wavelength 1.54184 Å. The Quanta 200 FEG scanning electron microscope was used to observe the morphology of the particles. Fourier Transform Infra-Red (FT-IR) spectra were recorded using Schimadzu Perkin-Elmer Spectrum FT-IR instrument with KBr pellets in the range 4000-450 cm–1. Magnetic properties of the samples were measured using Lakeshore VSM 7410 model.
Figure 1 shows the XRD patterns of lanthanum doped calcium hexaferrite with composition CaLaxFe12-xO19 (x= 0.5, 1.0, 1.5, 2.0).

Figure 1. XRD Pattern of CaLaxFe12-xO19
It was observed that the intensities vary with substitution. For the substitution x=0.5 and x= 1.0, the strongest peak appeared at (107) while for x=1.5 and x=2.0, it appeared at (110).
The average crystallite size D was determined from the position of the highest diffraction peak using the well known Scherrer equation, with a constant k of 0.9 (Shepherd, Mallick, & Green, 2007).
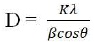
where β is the peak width at half maximum, θ is the peak position, and λ is wavelength of Cu-Kα radiation.
The X-ray density was determined with the help of following expression (Azim et al., 2014).
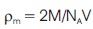
where 'M' is molecular weight of the composition and the factor 2 is for two molecules of the material contains in one unit cell. 'NA' is Avogadro's number and 'V' is volume of the unit cell. The structural properties of the samples are given in Table 1.
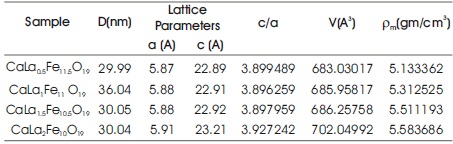
Table 1. Structural Parameters La Substituted Ca Hexaferrite (CaLaxFe12-xO19)
It was observed that the lattice constants a and c increase with increase in La3+ ion substitution. The variation in lattice constant c is more as compared to lattice constant a. The lattice volume and x-ray density also increases with increase in La3+ ion substitution. The substituted La3+ (1.16 Å) ion replaces Ca2+ (1.12 Å) ion in CaLaxFe12-xO19. To compensate the charge, some Fe3+ (0.645 Å) ions were converted into Fe2+ (0.780 Å) ions. The changes in lattice parameters may be attributed to differences in ionic radii of Ca2+ and La3+ ions (Kaur et al., 2017).
According to Verstegen and Stevels, c/a parameter ratio may specify the pattern type as M-type pattern if c/a ratio is less than 3.98. The observed values of c/a ration lies between 3.89-3.92, which are well within the range of M type of hexaferrite (Rehman et al., 2018).
The micrographs of the Ca-hexaferrite nano-particales with chemical composition CaLaxFe12-xO19 (x=0.5, 1.0. 1.5,2.0) calcinated at 950 oC has been displayed in Figure 2.
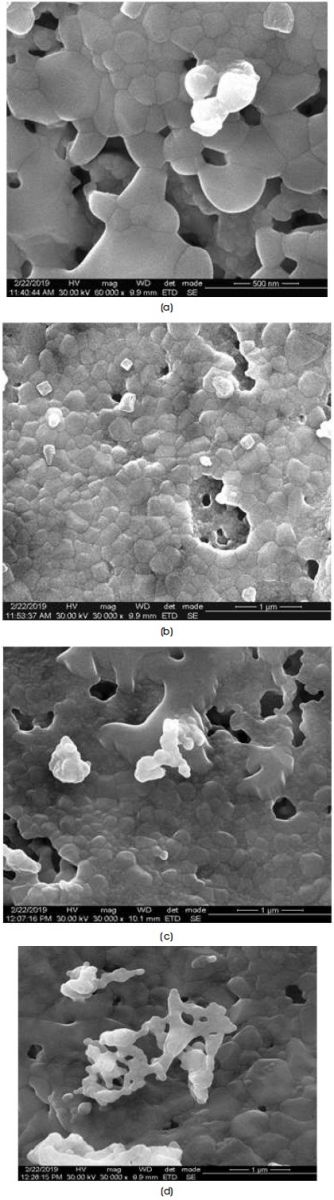
Figure 2. FE-SEM Micrographs of Calcium Hexaferrite a) CaLa0.5Fe11.5O19 b) CaLa1Fe11O19 c) CaLa1.5Fe10.5O19 d) CaLa2Fe10O19
SEM images show that the grains are in hexagonal platelet like morphology. With increase in La ion concentration, the grain size changes from 168 to 259 nm. The crystallite size calculated from the XRD data is smaller than the grain size derived from the SEM. This suggests that every grain is formed by aggregation of a large number of crystallites (Roohani, Arabi, Sarhaddi, & Sudkhah, 2017). EDAX spectrum for CaLaxFe12-xO19 (x=0.5, 1.0. 1.5, 2.0) powder is shown in Figure 3.
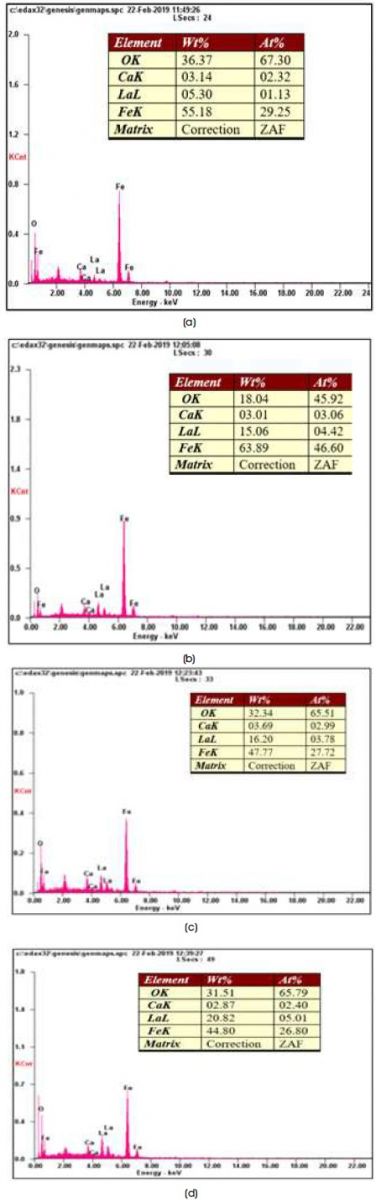
Figure 3. EDAX Spectrum of Calcium Hexaferrite a) CaLa0.5 Fe11.5O19 b) CaLa1Fe11O19 c) CaLa1.5Fe10.5O19 d) CaLa2Fe10O19
The composition of La and Fe and their molar ratios are shown in Table 2.
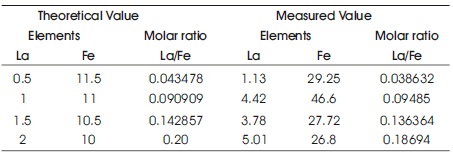
Table 2. Theoretical and Measured Value from EDAX Spectrum
The La/Fe molar ratios were found close to the theoretical value, which is a proof of homogeneous distribution of the elements in the solid. The EDAX patterns confirm the homogeneous mixing of Ca, La, and Fe atoms in samples and the purity of the chemical compositions (Rezlescu, Rezlescu, Popa, Doroftei, & Ignat, 2013).
Figure 4 shows the FT-IR spectra of calcinated powder of La substituted calcium hexaferrite for various La concentrations.
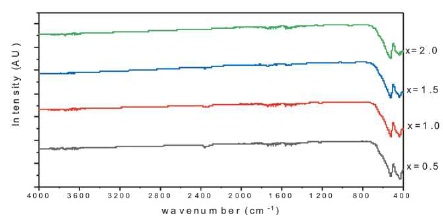
Figure 4. FT-IR Spectra of La Substituted Calcium Hexaferrite
It was also observed that the minimum % transmittance occurs for 432.062 cm-1, 435.919 cm-1, 520.78 cm-1, and 521.75 cm-1 for the La ion concentration 0.5, 1, 1.5, and 2.0, respectively. The IR spectra of all hexaferrites show the two principle absorption bands at 440 cm-1 and 520 cm-1. These two vibration bands are corresponded to the intrinsic lattice vibrations of octahedral and tetrahedral cluster in the hexaferrite. The absorption band between 520 and 440 cm-1 confirm the formation of hexaferrite (Dafe & Salunkhe, 2015). The band at 2350 cm-1 is due to free CO2 coming from citric acid (Bhat & Want, 2016). The band around 1620 cm-1 due to O–H bending band of the H2O molecules are chemically adsorbed to the magnetic particle surface (Najafabadi, Mozaffarinia, & Ghasemi, 2015).
Typical room-temperature hysteresis loops for samples CaLaxFe12-xO19 (x= 0.5, 1.0, 1.5, 2.0) prepared using sol gel auto combustion method are shown in Figure 5.
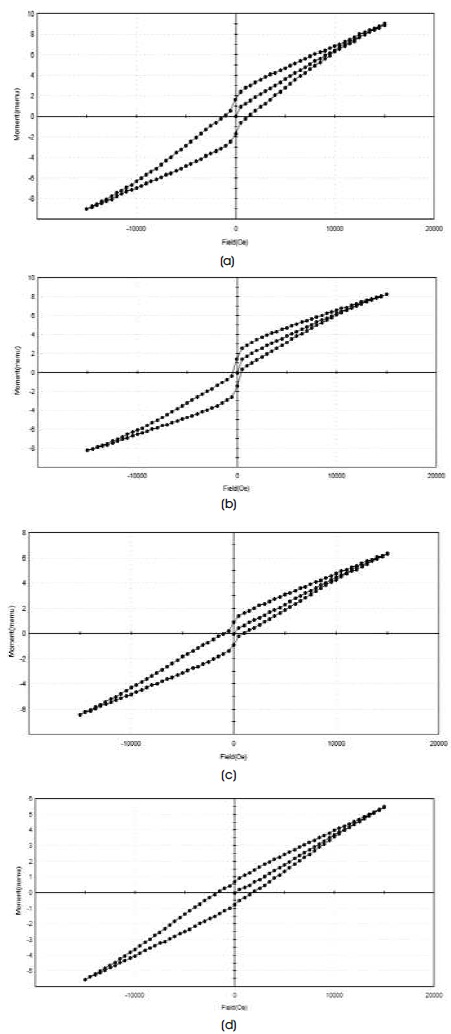
Figure 5. Hysteresis Loop for CaLaxFe12-xO19 Nano-Particle a) CaLa0.5Fe11.5O19 b) CaLa1Fe11O19 c) CaLa1.5Fe10.5O19 d) CaLa2Fe1019
The values of the saturation magnetization (Ms), coercivity (Hc), remanence (Mr) as obtained from hysteresis loop data are shown in Table 3.
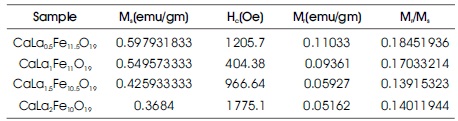
Table 3. Magnetic Parameters of La Substituted Calcium Hexaferrite
It was observed that as La ion concentration increases the remanence and saturation magnetisation decreases while the coercivity increases from 1205.7Oe to 1775.1Oe. Magnetic properties of Ca hexaferrite can be changed with substitution for Fe3+ or Ca2+ ion. As stated earlier, La3+ ion replaces the Ca2+ ion (2a site) in the structure of calcium hexaferrite. To compensate the charge, some Fe3+ ions get converted into Fe2+ ions. The magnetic moment of Fe3+ and Fe2+ are 5 μB and 4 μB, respectively (Guo et al., 2014). Also the super exchange interaction of 12K and 2a site (Fe3+ –O2- –Fe2+) becomes weaker (Wang et al., 2016). The combined effect is that the saturation magnetisation decreases with increasing La3+ ion concentration, The coercivity increases due to increase in magneto crystalline anisotropy constant (Qiao, Zhou, Zheng, Ying, & Che, 2016). Rashad, El-Sayed, Rasly, Sattar, and Ibrahim (2013) reported that the value of coercivity decreases with increasing the crystallite size (Rashad et al., 2013). It was found that the crystallite size increases from 29.99 nm to 36.04 nm for La ion concentration 0.5 to 1. Hence the coercivity was decreased from 1205.7Oe to 404.38Oe. Thereafter, the crystallite size decreases while the coercivity increases. The crystallite size as obtained by Scherer's formula was found to be 30 nm -36 nm. These sizes are suitable for use in magnetic recording media, which require sufficiently high coercivity (Ghanbari, Arab, Bor, & Mardaneh, 2017). For longitudinal magnetic recording medium, high coercivity up to 600Oe is required. Coercivity value above 1200Oe, the material can be used for the perpendicular recording media (Singh et al., 2016). So the prepared hexaferrite material can be suitable for longitudinal and perpendicular magnetic recording media.
The ratio of Mr and Ms measures a squareness ratio (SQR) of the hysteresis loop. For single domain magnetic structure of the sample, SQR should be less than 0.5. In these results shown in Table 3 reveal that all the samples have SQR less than 0.5 confirms the single domain structure for all the samples (Satone & Rewatkar, 2015).
In the present research, La substituted Ca hexaferrite is synthesized by auto combustion sol gel method. The XRD data confirm the formation of single magneto-plumbite phase belongs to space group P63/mmc. The lattice parameters a and c, lattice volume and x-ray density increases with increase in La ion substitution. The FIIR data also confirm the formation of hexaferrite. SEM micrograph gives the grain size in nano range. EDAX spectrum gives the homogeneous distribution of the elements in the solid. Magnetic properties, such as saturation magnetisation, coercivity, and remnant magnetisation changes with substitution. Hysteresis loop confirms the single domain structure for all the samples. The coercivity value changes from 1205.7Oe to 1775.1Oe make material suitable for the longitudinal and perpendicular magnetic recording media.