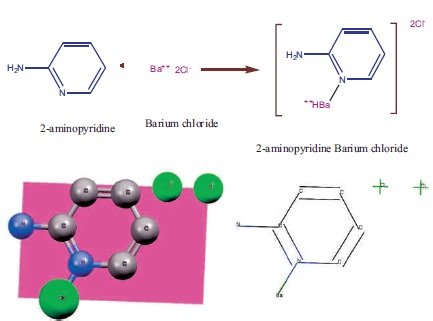
Figure 1. Molecular Scheme and ORTEP Diagram of 2APBC
The growth of 2-aminopyridine barium chloride (2APBC) crystal and its optical properties are discussed. 2APBC crystal was grown by slow evaporation solution growth technique at room temperature. FTIR analysis is effectively used for identifying the different molecular bonding and information about functional groups present in the synthesized compound. The powder sample of 2APBC crystal was used in Powder X-Ray Diffraction (PXRD) analysis to confirm the good crystalline nature of the sample. Single crystal X-ray diffraction analyses of grown crystal shows the unit cell lattice parameters value α = 5.281 , β = 5.410 , γ = 14.898 Å, α =β = γ=90º and volume V= 425.638 Å3, and confirms that 2-aminopyridine barium chloride (2APBC) crystal belongs to the orthorhombic crystal system with noncentrosymmetric space group P212121. Defect less; good quality 2APBC crystal was subjected to linear optical study. UV-vis-NIR spectroscopy study of the grown 2APBC crystal shows good transparency in the entire UV-vis-NIR region with lower cutoff wavelength 383 nm. Optical energy band gap (Eg) was calculated using UV spectrum data. Thermal behaviour of 2APBC sample was carried out using TGA and DTA analysis. The non-linear optical efficiency such as second harmonic generation was measured for 2APBC crystal and the result is compared with known inorganic reference material KDP. The dielectric constant is high at lower frequencies and decreases with increase in frequency trend were observed in dielectric polarization study. Hardness values of the grown crystal were estimated by Vickers's microhardness test.
Nonlinear optics is a frontier field in science and technology which has found wide application in the field of communication, optical and optical storage devices, etc. (Guangcai, Minhua, Zongshu, & Dong, 1987; Xiaoming, Yunlan, & Ziqin, 1987). Nonlinear crystals usually have mechanical and thermal properties and are susceptible for damage during processing even though they have large nonlinear efficiency. Also it is difficult to grow large size optical quality crystal of these materials for device fabrications (Swaminathan & Irving, 1964; Roshan, Joseph, & Ittyachen, 2001; Nair et al., 2014; Arivuselvi & Kumar, 2016; Arthi, Ilango, Mercina, Jayaraman, & Joseph, 2017). The inorganic material arises from the strong charge transfer and polarizability (Arthi et al., 2017; Le Fur, Bagieu-Beucher, Masse, Nicoud, & Lévy, 1996; Gandhimathi, Krishnan, & Selvarajan, 2015). An inorganic crystals have excellent mechanical and thermal properties but posses' relatively modest optical nonlinearity because of the lack of extended π-electron delocalization (Liu et al., 2007; Maadeswaran, Thirumalairajan, & Chandrasekaran, 2010; Rose, Selvarajan, & Perumal, 2011).
The large second order electric susceptibility has concentrated on acentric organic (or) organometallic chromophores with an organic π-electron system coupling electron donor and acceptor group (Laidlaw, Denning, Verbiest, Chauchard, & Persoons, 1993). Many semiorganic nonlinear optical material have been grown by slow evaporation solution growth technique, which enhances the nonlinear optical field by involving one (or) more kind of hydrogen bond have been reported (Xue, & Zhang, 1999; Yu, Xue, & Ratajczak, 2006; Sinha et al., 2009). A semiorganic material is a combination of both organic and inorganic materials (Babu & Ramasamy, 2010; Rajkumar, Xavier, Anbarasu, & Devarajan, 2016). Many optically active organic amino acids are mixed with the inorganic salts in order to enhance their physical and chemical properties (Ganesh, Kannan, Sathyalakshmi, & Ramasamy, 2007). Currently the development of advanced new functional materials is of great interest.
Organic compound crystalline in centrosymmetric space group due to predominant antiparallel p-stacking between the aromatic ring as a consequence of dipolar interaction (Abrahams & Robertson, 1948; Donohue & Trueblood 1956). In addition to inorganic compound, the crystal crystallizes in non-centrosymmetric space group. This is particularly true for non-centrosymmetric a material that is compound crystallizing in a space group with no centre of symmetry (Balakrishnan & Ramamurthi, 2008).
Pyridine is a good hydrogen bond acceptor and strong organic base, the molecule 2-aminopyridine has often used as a ligand in metal complex (Qin et al., 1999) with nitrophenols to generate noncentrosymmetric lattice (Prakash & Radhakrishnan, 2005) that exhibits SHG efficiency. Aminopyridine ligand complexes are class of compounds well known for a long time (Srineevasan & Rajasekaran, 2013; Srineevasan, & Rajasekaran, 2014a; Srineevasan & Rajasekaran, 2014b). Metal complexes have attracted considerable attention owing to their application in second and third harmonic generation (Ding, Mu, & Gu, 2000; Menon, Philip, Deepthy, & Bhat, 2001; Dhanasekaran & Srinivasan, 2013). Materials containing non-centrosymmetric often exhibit important technological properties such as second harmonic generation (Misoguti et al., 1996)
Some of the researchers reported promising 2- aminopyridine derivatives are, 2-maleic acid (Dhanaraj, Rajesh, & Bhagavannarayana, 2010) 2-aminopyridine pamino benzoic acid (Periyasamy, Jebas, Gopalakrishnan, & Balasubramanian, 2007) 2-aminopyridine p-nitro benzoic acid (Periyasamy, Jebas, & Thailampillai, 2007), 2-aminopyridine 4-nitrophenol (Babu, Ramasamy, & Ramasamy, 2009), 2-aminopyridine silver nitrate (Bhuvana, Jebas, & Balasubramanian, 2010), 2 – aminopyridine 5-chloropyridinium L-tartrate (Jayanalina, Rajarajan, Boopathi, & Sreevani, 2015), 2-aminopyridine potassium chloride (Srineevasan & Rajasekaran, 2014a), 2-aminopyridine thiourea zinc sulphate (Srineevasan & Rajasekaran, 2013), 2-aminopyridine potassium thiocyanate glycine (Srineevasan & Rajasekaran, 2014b), 2-amino-5 nitropyridinium chloride (Zaccaro, Capelle, & Ibanez, 1997) 2-amino-5 nitropyridinium L-monohydrate tartrate (Pécaut & Masse, 1994) are available in literature and are good candidate for NLO applications.
In the present work, efforts have been made on growth of 2-Aminopyridine Barium Chloride (2APBC) non-centrosymmetric crystal by slow evaporation solution growth technique at room temperature. The grown crystal was analyzed with various characterization and the physicochemical properties are discussed in detail.
Equimolar ratio (1:1) of high purity 2-aminopyridine (with Molecular weight 94.11 g/mol, AR Grade, Sigma Aldrich) and barium chloride (with Molecular Weight 208.23 g/mol, AR Grade, Sigma Aldrich) were taken and dissolved in Millipore water at room temperature to synthesis 2-Aminopyridine Barium Chloride (2APBC). Synthesized compound of 2APBC has been done by using the chemical reaction in Figure 1.

Figure 1. Molecular Scheme and ORTEP Diagram of 2APBC
The purity of the synthesized salt was further improved by successive recrystallization process. The solubility of the synthesized salt was carried out at various temperature 30, 35, 40 and 45 ºC in 5 ºC intervals by dissolving the solute in Millipore water in an airtight container maintained at constant temperature with 6 hours stirring using magnetic stirrer. After the saturation point, the equilibrium concentration of the solution was analysed gravimetrically. The same procedure was repeated and the solubility curve for different temperature was drawn. Figure 2 shows the solubility curve of 2APBC in aqueous solution. It is observed from the curve that the crystal posses positive solubility gradient, which is used to grow good optical quality crystal by slow evaporation.
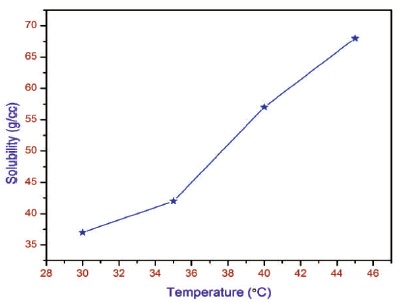
Figure 2. Solubility Curve of 2APBC Crystal
The saturated solution 2-aminopyridine barium chloride (2APBC) was filtered using good quality Whatmann filter paper to remove the unsolvable impurities. Then the filtered solution was transferred into beaker and sealed with porous paper with few holes. The beaker was kept in an undisturbed and dust free atmosphere. After the time period of 65 days the tiny seed crystals were observed.
Macroscopic defect free transparent crystals were selected as seed for growing large size single crystal of 2APBC. The seed crystal is introduced into the mother solution. The crystal was grown as a bulk crystal with dimension 12 mm × 7 mm × 4 mm in the period of 85 days. As grown crystal of 2APBC is shown in the Figure 3.
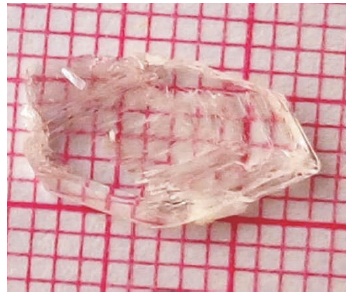
Figure 3. As Grown 2APBC Crystal
The infrared spectral analysis is effectively used to understand the chemical bonding and it provides information about molecular structure of the synthesized compound (2APBC) by using Thermo Nicolect v-200 FTIR spectrometer by KBr pellet method in the range 500-4000 -1 cm . Freshly crushed powder of 2APBC crystal was subjected to FTIR study. FTIR spectrum of 2APBC crystal is shown in Figure 4 and the observed frequencies and their assignment are shown in the Table 1. A peak observed at 2779 cm-1 is due to C-H stretching. The sharp peak observed at 2735 cm-1 due to NH stretching vibration. The 2 bands appear in the region 2395 cm-1 is assigned for NH stretching. The split band at 2065 cm-1 and 1382 cm-1 is due to ligand coordination (Schilt & Taylor, 1959). The resolved sharp peak at 1762 cm-1 is due to C-H stretching.
N-H bending vibration was identified at 1664 cm-1. NH2 rocking vibration was found at 1552 cm-1. The out of plane bending vibration (=C-H) was identified at 852 cm-1. The intense out of plane CH deformation mode of substituted pyridine occurs at 700 cm-1 to 800 cm-1 region (Katritzky, 1959). A peak at 551 cm-1 is due to C=C-C out of plane ring and 437 cm-1 is due to C=C plane ring bending. A sharp peak observed at 408 cm-1 is identified for Cl stretching. The assignments confirm the presence of various functional groups in the material.
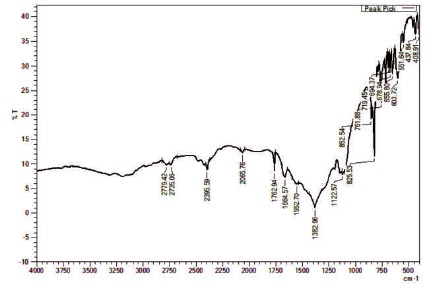
Figure 4. The FTIR Spectrum of 2APBC Crystal
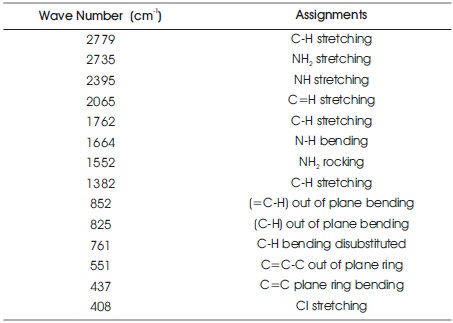
Table 1. Wave Number Assignments of 2APBC Crystal
Powder XRD diffraction analysis was carried out using BRUCKER, Germany (model D8 Advance) X-ray diffractometer with CuKalpha (wavelength = 1.5405 Å) radiation. The powder sample (2APBC) was scanned over a range 10 to 80o at a scanned rate of 1o per minute to study the crystalline nature of the grown crystal. The well defined sharp Braggs peak signifies the good crystalline nature of 2APBC crystal. The hkl values are fixed using INDX Software. The diffracted peaks are shown in Figure 5.
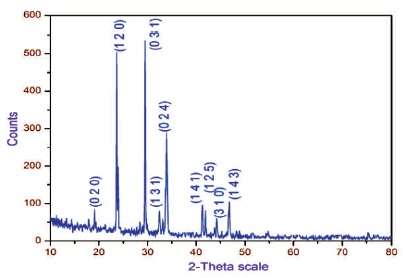
Figure 5. Powder XRD Pattern of 2APBC Crystal
Single Crystal X-ray diffraction analysis of 2-Aminopyridine Barium Chloride (2APBC) crystal was recorded using ENRAF NONIUS CAD-4 automatic X-ray diffractometer. Unit cell lattice parameter values of 2APBC crystal are a = 5.281 Å, b = 5.410 Å, c = 14.898 Å, α =β = γ=90º and volume V= 425.638 Å3; this analysis reveals that the 2APBC crystal cr ystallizes in orthorhombic system with noncentrosymmetric space group 212121. The incorporation of barium in 2APBC crystal was confirmed by changes in crystal system by comparing with reported values of 2AP (Chao, Schemp, & Rosenstein, 1975) shown in Table 2.
The optical transparent spectrum was recorded using DOUBLE BEAM UV-vis Spectrophotometer: 2202 in the region 200-900 nm and the optical transmittance spectrum of 2-Aminopyridine Barium Chloride (2APBC) is shown in Figure 6. In 2APBC crystal, the UV cut-off wavelength found at 383 nm and the percentage of transmission is high in the entire visible region from 383 nm to 800 nm, there is a sharp decrease in transmittance, due to absorption leading to excitation in this region.
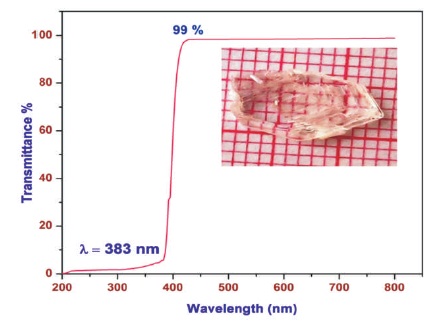
Figure 6. Optical Transmittance Spectrum of 2APBC Crystal
Near UV region absorption arises from electronic transition associated with in 2-aminopyridine, the orbital π-electron delocalization arises from the mesomeric effect. This π-electron delocalization is responsible for its nonlinear optical properties and absorption in near UV region (Ushasree, Jayavel, & Ramasamy, 1999). The wide range of transparency in grown crystal is an added advantage in the field of optoelectronic applications (Ganesh et al., 2007) Optical energy band gap (Eg=4.1 eV) was shown in Figure 7.
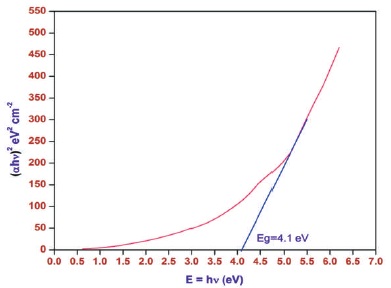
Figure 7. Tauc's Plot of 2APBC Crystal
Thermal properties of 2APBC crystal was studied by Thermogravimetric (TGA) and Differential Thermal Analysis (DTA) using STA 409 C instrument. TG-DTA curve of 2APBC crystals was shown in Figure 8. DTA curve shows a sharp endothermic peak at 169ºC for 2APBC, which corresponds to the melting point of the compound.
Hence, the thermal stability of 2-aminopyridine barium chloride is 169 ºC. Due to the addition of barium the thermal stability is increased. The material decomposes at 321 ºC is represented by the sudden loss of mass. From the TG curve, the mass loss takes place after the temperature of 321 ºC. The mass lost from 169 ºC to 321 ºC is found to be 43%. The actual residual amount of mass is 43% which may be considered to be the compound of barium.
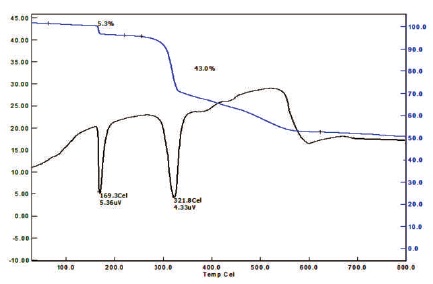
Figure 8. TG and DTA Curve of 2APBC Crystal
The powder SHG measurement was performed using a modified Kurtz and Perry technique with (λ=1064 nm) IR laser radiation (Kurtz & Perry, 1968). The powder sample of 2APBC with an average particle size range 125-150 μm is filled in a micro-capillary tube about1.5 mm diameter. To make relevant comparision with known SHG materials, Potassium dihydrogen phosphate (KDP) was also filled in a micro-capillary tube with same range. The SHG output (λ=532 nm) green light emission was collected by a photomultiplier to collect only the green radiation. The input laser energy incident on the capillary tube was chosen to be 0.68 J and the output power from the powder sample of 2APBC and KDP was measured to be 8.3 mJ and 8.8 mJ. The output power 8.3 mJ was compared with output 8.8 mJ (Figure 9) of known KDP. The bright green radiation (Figure 10) of wave length λ=532 nm (P2ω) by the sample confirms Second Harmonic Generation (SHG). The powder SHG efficiency of 2APBC crystal was confirmed by emission of green light. It is observed that the frequency doubling efficiency of 2APBC crystals was originate to be 0.94 times greater than KDP; it is evident that 2APBC crystal was suitable for NLO device fabrications.
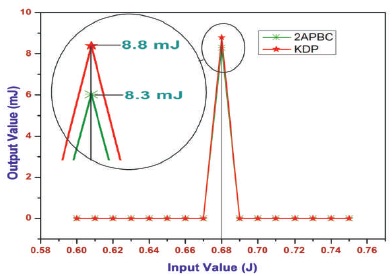
Figure 9. SHG Efficiency of 2APBC Crystal
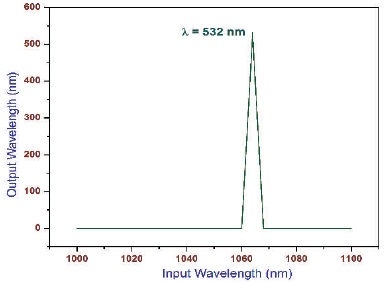
Figure 10. SHG Emission Spectrum of 2APBC CRYSTAL
The dielectric constant and the dielectric loss of the 2APBC sample were measured using HIOKI 3532-50 LCR HITESTER. The measurement has been done as a function of frequency. The presence of dielectric between the capacitor plates increases the capacitance of the capacitor. Good quality crystal of dimension 5 x 4 x 2 mm3 were polished and electronic grade silver paste was applied on either side of the 2APBC crystal, which act as an electrode. Dielectric studies were carried out in the temperature range 308 K to 348 K in the varying frequency (Smyth, 1965). Dielectric constant of the crystal was calculated using the relation,
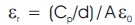
where, Cp - Parallel plate Capacitor,
A - Overlap of two plates,
d - Separation between the plates.
The high value of dielectric constant at low frequency may be due to the presence of all the polarizations namely, space charge, orientation, ionic and electronic polarization (Dharmaprakash & Rao, 1989). At low frequency only space charge polarization is active. The inactive of other three polarization may be lead to low value of dielectric constant at high frequency (Singh et al., 2010). The variation of dielectric constant as a function of frequency for different temperatures is shown in Figure 11. The variation of dielectric loss as a function of frequency for different temperatures is shown in Figure 12. The dielectric loss decreases initially with frequency and then attains a constant. Similar trend is observed for other temperatures in both dielectric constant and dielectric loss measurements.
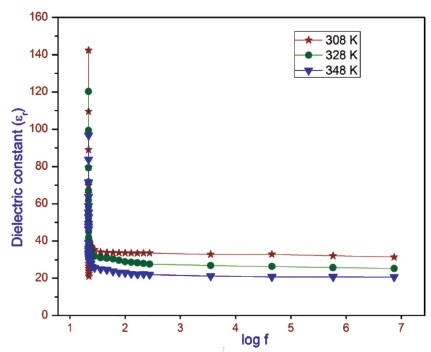
Figure 11. Variation of Dielectric Constant with Log Frequency at Different Temperatures for 2APBC Crystal
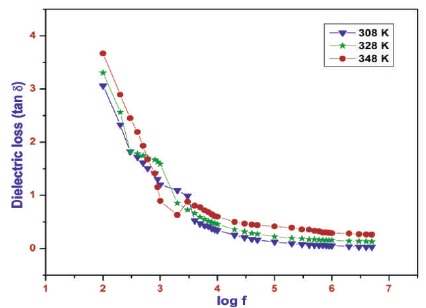
Figure 12. Variation of Dielectric Loss with Log Frequency at Different Temperatures for 2APBC Crystal
The grown 2APBC crystal was subjected to microhardness test using Leitz Wetzlar microhardness tester fitted with Vicker's pyramidal indenter and attached to an optical microscope (Mott, 1956; O'Neill, 1967). Several indentations were made for each load and the average value of the diagonal length was used to calculate the microhardness number (Hv). Figure 13 shows the variation of microhardness number with applied load and it is noted that hardness value increases with increase of load. Cracks were initiated on the crystal surface around the indentation for the applied load 100 g. In order to know the hardness nature of the material, the work hardening coefficient (n) was determined from the plot between log p and log d (Figure 14) by the least square fit method (Onitsch, 1956). The value of 'n' is found to be 1.0, thus indicating the hard nature of 2APBC crystal. The linear graph was observed within the slope of 1.6 which confirms the high mechanical strength of the crystal (Madhavan et al., 2007).
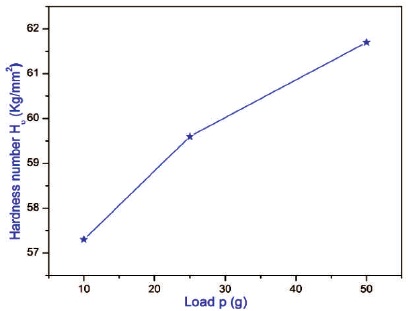
Figure 13. Variation of Load (p) with Hardness (Hv) for 2APBC Crystal
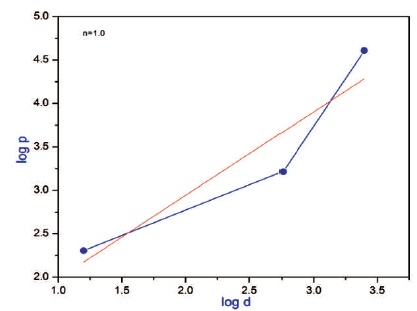
Figure 14. Variation of Log (d) with log (p) of 2APBC Crystal
2.9 Elastic Stiffness Constant (C11)
The elastic stiffness constant (C11) gives an idea about tightness of bonding between neighboring atoms (Dharmaprakash & Rao, 1989; Babu, Bhagavannarayana, & Ramasamy, 2008). The stiffness constant for different loads has been calculated using Wooster's empirical formula and is shown in Figure 15.

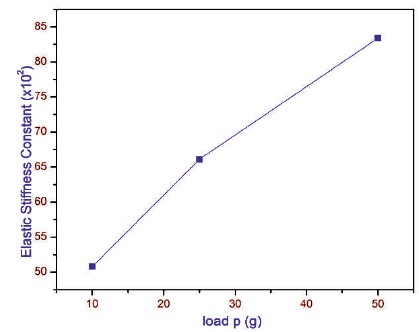
Figure 15. Variation of Load (p) with Stiffness Constant of 2APBC Crystal
Transparent and defect free crystal of 2APBC were harvested in the period of 85 days using slow evaporation technique at room temperature. The presences of functional groups in the grown crystal 2APBC were identified by FTIR analysis. The sharp and well defined Bragg peaks observed in the powder XRD pattern confirm the crystalline nature of 2APBC crystal. Single crystal XRD analysis revealed that the crystal system belongs to orthorhombic crystal system. The UV cut-off wavelength was found to be 383 nm. Optical band gap energy was found to be Eg=4.1 eV for 2APBC crystal. A thermal property of the material was studied by Thermogravimetric (TGA) and Differential Thermal Analysis (DTA). Second Harmonic Generation (SHG) of powdered 2APBC sample was tested using Nd: YAG laser and is found to be 0.94 times that of potassium dihydrogen orthophosphate (KDP). The dielectric study was carried out as a function of frequency at different temperature. The mechanical strength of the 2APBC crystal was evaluated and it describes that the Vickers hardness number of 2APBC is found to be increase with the applied load. The work hardening co-efficient (n=1.0) has been calculated from the slope of straight line between log P and log d. The stiffness constant for different loads has been calculated.