Figure 1. Classification of Corrosion
Hot corrosion is a major problem in engines, steam generators, steam turbine, and thermal power plants. The coating is one of the effective methods to reduce hot corrosion. However, better understanding of the hot corrosion performance of thermal spray coating on a different substrate at different temperature and environment, is required to reduce maximum hot corrosion. This paper summarizes the behavior of Ni, Co, Fe, and metal oxide based coating in the simulated boiler environment. The consolidated results of coating properties, such as coating hardness, porosity, thickness, and parabolic constant has also been detailed with emphasis on corrosion and its mechanism.
Corrosion is the unexpected gradual degradation of a metallic element by its chemical reaction with the surrounding environment. As a consequence of corrosion, metallic elements get changes to its chemical compounds like oxides, nitrides, chlorides, and sulphides depending upon the surrounding environment. The gradual degradation of a metallic element changes the properties of materials, which must be preserved (Sidhu et al., 2006a). The formation of rust and green film on the metallic surface is an example of corrosion. Losses due to corrosion could be about two lakh crores per year in India. Corrosion has a great economic and environmental effect on all aspects of countrywide infrastructure, such as highways, bridges, sewage, tunnel, buildings, oil and gas industries, chemical processing, and basically on all metal components in use.
Erosion-corrosion alone has been reported to be responsible for 50-75% of the total arrest time in such applications, and accounts for multi-million dollar loss to the relevant industries (Hidalgo et al., 1997). Advances in materials development and cooling schemes will lead to increased operating temperatures of steam turbine, gas turbines, fluidized bed combustion, and industrial waste incinerators (Khanna and Jha, 1998). The collaboration of such elevated temperatures with the simulated boiler environment such as Na2SO4 -60%V2O5 and low-grade fuels, such as Sodium (Na), Sulphur (S), Vanadium (V), and Chlorine (Cl), need special attention to the phenomenon and mechanism of hot corrosion (Eliaz et al., 2002). This type of corrosion consumes the material at an unpredictabe rapid rate that can be determined by the parabolic rate constant. The lower value of the parabolic rate constant (Kp) of coated specimen as compared with bare specimen, indicates that the coated layer acts as a mass barrier against the outside diffusion of cations, mainly Chromium (Ebrahimifar and Zandrahimi, 2011). Lower value of the parabolic rate constant indicates that there is more oxidation resistance. During cyclic oxidation, the lower value of Kp of coated samples may be due to good p resistance against spallation and cracking. Premature failure of high performance machinery such as boiler is due to hot corrosion. This leads to large economic losses.
According to the environment, corrosion is classified into two categories; Dry Corrosion and Wet Corrosion, as shown in Figure 1.

Figure 1. Classification of Corrosion
Dry Corrosion
Dry corrosion is also known as chemical corrosion in which metallic objects get degraded due to direct chemical attack of metal surfaces in the presence of atmospheric gases, such as H, He, N, SO , etc. Dry corrosion occurs in the 2 absence of moisture or water. Degradation of steel by furnace gases is an example of dry corrosion. Dry corrosion is further divided into oxidation corrosion, liquid metal corrosion, and corrosion by other gases.
Mechanism:
Oxidation appears at the metal surface, forming metal ions M 2+
M →M2++ 2e-
Oxygen is transformed to oxide ion (O2- ) owing to the shift of electrons from a metallic element
n/2 O2 + 2ne- →nO2-
The overall reaction is of oxide ion reacts with the metal ions to form a metal oxide film.
2 M + n/2 O2 →2Mn+ + nO2-
Simply, Metal + Oxygen Metal oxide (corrosion product)
Wet Corrosion
Wet Corrosion is also known as electrochemical corrosion which occurs when a liquid is present. It is due to the flow of an electron from a metallic surface anodic area towards the cathodic area through a conducting medium. Corrosion of steel by water (H2O) is an example of wet corrosion.
Wet corrosion is further classified into bimetallic corrosion and concentration cell corrosion.
Concentration cell corrosion takes place when one part of metallic component is exposed to a different air concentration from the other part. For example, if a metallic component is partially immersed in a conducting medium/solution, the metal component above the solution is more aerated and becomes cathodic. The metallic component in the medium/solution is less aerated and thus becomes anodic and suffers corrosion.
Mechanism:
At anode: Corrosion takes place (less aerated) M→ M2+ +2e-
At cathode: OH ions are produced (more aerated), ½ O2 + H2O + 2e- →2OH-
Hot Corrosion
High temperature corrosion was first recognized as a serious problem in the 1940s in connection with the degradation of fireside boiler tubes in coal-fired steam generating plants. Since then the problem has been observed in boilers, military aircrafts, internal combustion engines, gas turbines, fluidized bed combustion, and industrial waste incinerators. According to Rapp (1986), “Hot corrosion has been defined as the accelerated oxidation of materials, at elevated temperatures, induced by a thin film of fused salt deposit”. Srikanth et al. (2003) have reported that the fireside corrosion in a boiler occurs due to the reaction of sulphur species in the gas phase with metal surfaces. The sulphur present in coal and fuel oils, especially in low-grade fuels, yields SO2 on combustion, which is partially oxidized to SO3 . The NaCl (either as impurities in the fuel or in the air) reacts with SO3 and water vapour at combustion temperatures to yield Na2SO4 (melting point 884 C) (Natesan, 1976). Small amount of vanadium may also be present in fuel oils which on combustion form V2 O5 (melting point 670 0C). This may further react with Na2SO4 to form low melting sodium vanadates, which are extremely corrosive to high temperature materials used in the combustion system (Hwang and Rapp, 1989).
It has been concluded that the better understanding of hot corrosion performance of thermal spray coating properties on a different substrate at different temperature and environment is required to reduce maximum hot corrosion, so that the life of high performance machinery, such as boilers, steam turbines, and gas turbines, subjected to high temperature environments increases.
Nickel-chromium and Cobalt-chromium based alloys are widely used as coating materials due to their several attractive properties, such as wear, erosion and corrosion resistance, and good thermal conductivity. Due to these properties, Ni-Cr coatings are frequently considered to control the problem of erosion-corrosion of power plant boilers (Wang et al., 2002). This paper summarizes the parabolic rate constant and coating properties of different spray methods for 50 cycles in the simulated boiler environment at 900 0C and 700 0C.
Hot corrosion and erosion in coal fired steam generator at elevated temperature is the primary reason behind downtime in power generating plants. In actual coal fired boiler power plant, it is found from the literature that approximately 89 failures occur in an year, out of which 50 failures are due to hot erosion and corrosion process, resulting in economic losses (Prakash et al., 2001). The hot corrosion and erosion is a synergistic process, which is mainly occuring in coal fired steam generator, gas turbine, steam turbine, I.C. engines, incinerators, pump impeller, biomedical, naval, electronics, and other related components subjected to very high temperature. These problems can be overcome by either changing the material or changing the environment or by separating the material surface from the environment. The distinct investigations have been carried out to overcome the erosion problems and found that erosion resistant coating is an effective way to resist corrosion in a coal fired boiler. Various authors deposited Fe, Ni, and metal oxide based coating on a different substrate at 900 0C by thermal spray processes. From the value of % age reduction in the parabolic rate constant and coating properties of different thermal spray processes, Ni-Cr and Stellite-6 coating reduce maximum hot corrosion. Sidhu et al. (2006a) investigated the hot corrosion behaviour of plasma spray and laser remelted Stellite-6 (Cobalt and chromium alloy) coating on GrA1, T-11, and T-22 steel in Na2SO4 –60%V2 O5 at 900 0C for 50 cycles. From the result of SEM (Scanning Electron Microscope)/ EDAX (Energy Dispersive Analysis of X Rays), X-RD (X-ray diffraction), and EPMA (Electron-Probe Micro-Analysis), it was found that stellite-6 coating on GrA1, T-11 and T-22 steel reduces hot corrosion to 95%, 88%, and 86%, respectively. It means cobalt-chromium based coating (Stellite-6) on GrA1 is also effective in reducing hot corrosion. The thermo-gravimetric technique was used to study the weight change data. To establish corrosion behaviour, weight change for coated and uncoated steel 2 (mg/cm2 ) and square of total weight gain value per unit area (mg2/cm4 ) are depicted in Figures 2 and 3, respectively.
SEM back scattered micrographs for plasma sprayed stellite-6 and laser remelted steel after 50 cycles at 900 oC are shown in Figure 4 (a-e). In Laser remelted steel bare specimens, the corroded species entered are shown in Figure 4 (d and e) and minimum scale was shown in case of Stellite -6 coated GrA1.
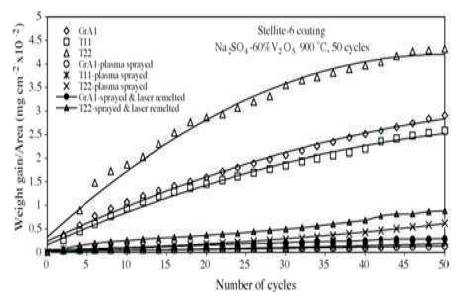
Figure 2. Weight Gain Plot for Uncoated and Stellite-6 Coated Steels with Bond Coat Exposed to Na2SO4 –60%V2 O5 at 900 oC for 50 Cycles (Sidhu and Prakash, 2006a)
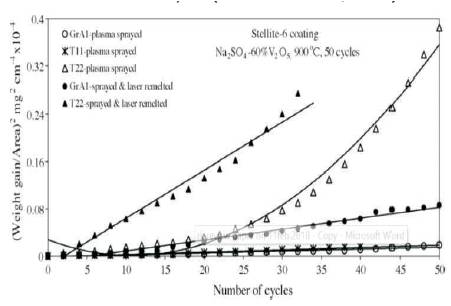
Figure 3. Weight Gain Square (mg /cm ) plot for Stellite-6 Coated Steels with Bond Coat Exposed to Na2SO4 –60%V2 O5 at 900 oC for 50 Cycles (Sidhu and Prakash, 2006b)
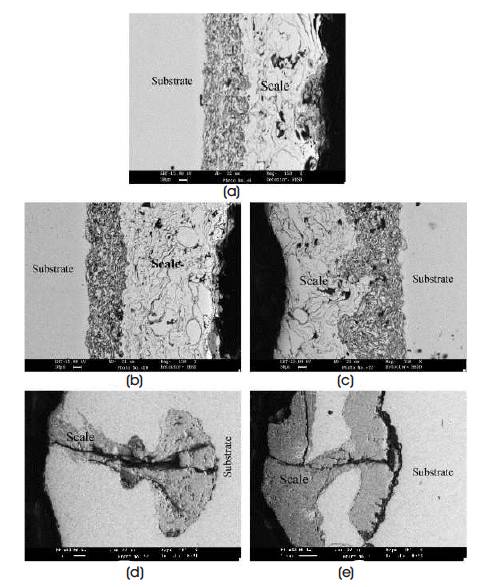
Figure 4. SEM Back Scattered Image for Stellite-6 coated Steels after Cyclic Hot Corrosion in Na2SO4 –60%V2O5 at 900°C for 50 Cycles: (a) GrA1, 150× (b) T11, 150×, (c) T22, 150×, (d) GrA1 Corroded after Laser Remelting, 200×and (e) T22 Corroded after Laser Remelting, 100×(Sidhu et al., 2006d)
The XRD shows the presence of cobalt and chromium oxides and their spinels as shown in Figure 5.
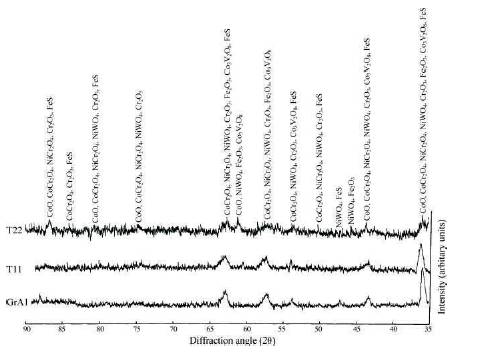
Figure 5. X-ray Diffraction Profiles for Stellite-6 Coated Steels with Bond Coat Exposed to Cyclic Hot Corrosion in Na2SO4 –60%V2 O5 at 900 oC for 50 Cycles (Sidhu et al., 2006c)
The main phases formed for stellite-6 coating were CoCr2O4 , Cr2 O3 , CoO, NiWO4 , Fe2O3 , FeS, and Co3V2O5 (Figure 6).
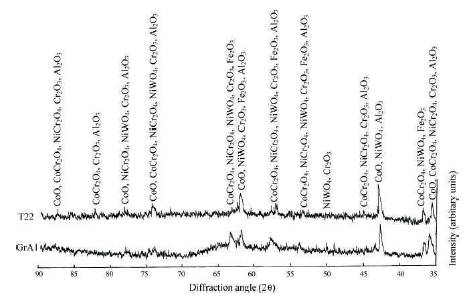
Figure 6. X-ray Diffraction Profiles for Stellite-6 Coated and Laser Remelted Steels with Bond Coat Exposed to Cyclic Hot Corrosion in Na2SO4 –60%V2 O5 at 900 oC for 50 Cycles (Sidhu et al., 2006f)
The investigator results show that stellite-6 and laser remelting coating is effective in decreasing hot corrosion and porosity, respectively.
Sidhu et al. (2006g) have successfully deposited Liquefied Petroleum Gas (LPG) assisted High Velocity Oxygen Fuel (HVOF) sprayed Ni-Cr based coating on boiler steel specimens, namely: GrA1, T-11, and T-22 respectively at 900 0C for 50 cycles. From the value of % age reduction in the parabolic rate constant for GrA1, T-11, and T-22 coatings were 99.91%, 99.67%, and 99.80%, respectively. The hot corrosion of bare and coated samples in (mg/cm2) were (349.32, 334.3, 426) and (10.58, 13.76, 20.2), respectively. The weight gain per unit area (mg/cm2) during hot corrosion for the bare and coated steel specimens in the presence of Na2SO4 –60%V2O5 environment at 900 0C is shown in Figure 7.
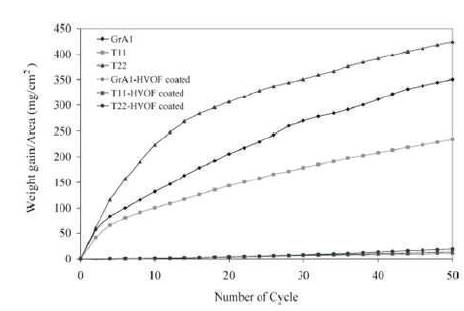
Figure 7. Weight Gain vs. Number of Cycles Plot for Coated and Uncoated Steels Subjected to Cyclic Oxidation for 50 Cycles in Na2SO4 –60%V2 O5 at 900 °C (Sidhu et al., 2006f)
Parabolic rate law, i.e. (Weight gain)2 vs. number of cycles for hot corrosion tests are presented in Figure 8.
From the Electron probe micro-analyzer (EPMA) analysis map for T-11, NiCr coated corroded steel, it is found that Iron (Fe) and Nickel (Ni) have diffused into the top of scale and into the substrate respected as depicted in Figure 9. Whereas optical micro -structure and surface appearance of uncoated and NiCr-GrA1 coated steel after cyclic oxidation for 50 cycles in molten salt environment is shown in Figure 10.
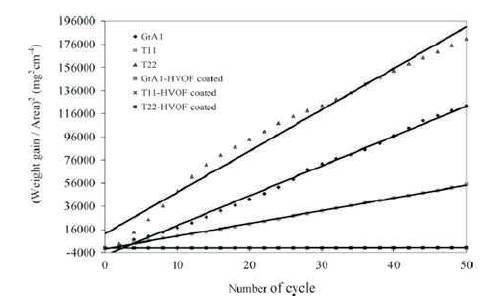
Figure 8. (Weight Gain)2 vs. Number of Cycles Plot for Coated and Uncoated Steels Subjected to Cyclic Oxidation for 50 Cycles in Na2SO4 –60%V2 O5 at 900 °C. Ni-Cr and Stellite-6 Coating Reduce Maximum Hot Corrosion (Sidhu et al., 2006f)
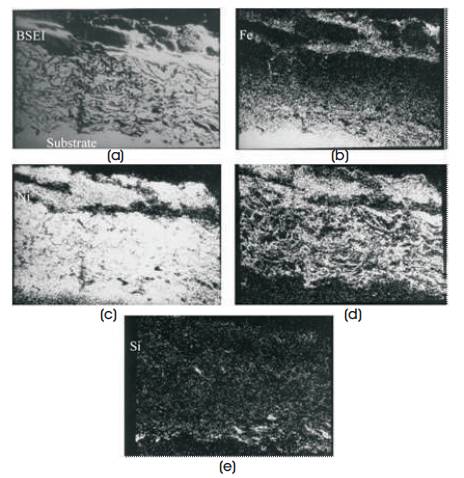
Figure 9(a-e). Composition Image (BSEI) and X-ray Mapping of the Cross Section of Ni-Cr Coated T11 Steel Subjected Cyclic Corrosion in Molten Salt at 900°C (Sidhu et al., 2006g)

Figure 10(a & b). Optical Microstructure along the Crosssection for NiCr as Coated GrA1 Steel at 200X and Surface Appearance of Uncoated and Coated Steels after Cyclic Oxidation for 50 Cycles in Na2SO4 –60%V2 O5 respectively (Sidhu et al., 2006g)
From the test results, it is found that HVOF sprayed LPG assisted NiCr coating on selected boiler steels, reduce maximum hot corrosion, which may be due to the formation of protective oxides like NiO, NiCr2O4 , and Cr2O3 . The results of hot corrosion behaviour in terms of parabolic rate constant at 900 oC before and after coating on different boiler steels along with coating properties are summarized in Table 1.
Table 1. Study of Parabolic Rate Constant and Various Properties of Ni, Fe, and Metal Oxide based Coating by Thermal Spray Techniques at 900°C for 50 Cycles in Simulated Boiler Environment. (CS: Cold Spray, PS: Plasma Spray, HVOF: High velocity-oxy fuel spray, D Gun: Detonation Gun Spray, HVAF: High Velocity-air Fuel Spray)
The results of hot corrosion behaviour in terms of parabolic rate constant at 700 0C before and after coating on different boiler steels along with coating properties are summarized in Table 2.
Table 2. Study of Parabolic Rate Constant and Various Properties of Ni, Fe, and Metal Oxide based Coating by Thermal Spray Techniques at 700°C for 50 Cycles in Simulated Boiler Environment
Hot corrosion is a major problem in thermal power plants, gas turbine for aircraft and ship, resulting in large economic losses. To avoid major economic losses, suitable corrosion resistance coating must be applied. Hence, a better understanding of the parabolic rate constant and coating properties of thermal spray coating is required to reduce maximum erosion corrosion. So far as the composition of coatings for boiler application is concerned, it is well proven fact from the literature survey that the Ni based coatings offer high temperature oxidation and corrosion resistance in fossil fuel coal fired boilers. This may be due to the formation of oxides and spinels of Ni, Al, and Cr.
The authors would like to thank the Management of Chandigarh Group of Colleges in Landran, Mohali (Punjab), India, and his brother Mr. Santosh Kumar for their continuous support to carry out this research review work.