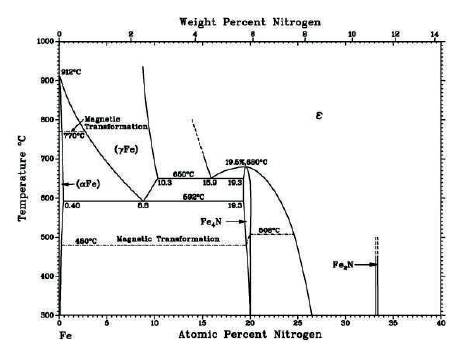
Figure 1. Iron-nitrogen Equilibrium Phase Diagram (Pye, 2003)
In this review paper, an effort has been made to understand the plasma ion nitriding process and other existing nitriding processes used in industries. The solubility of nitrogen atoms in the steel matrix have been explained through Fe-N system. The formation of different stable and metastable compounds during nitriding process has been discussed. Different types of nitriding processes have been thoroughly reviewed with their advantages and disadvantages. It was found that plasma ion nitriding process is more reliable as compared to other existing processes in use. Important results of diagnostics and its applications to improve the mechanical and chemical (corrosion) properties of ferrous and non ferrous alloys have been discussed. It was found that N2+, N+ , NH, Hα , and Hβ species exist in the plasma nitriding process, but nitrogen ions (N+) was the most dominant species. Hydrogen plays a significant role in the plasma nitriding process. It was reported that surface hardness and case depth were maximum for the gas ratio of 10% N2 and 90% H2 . The effect of various plasma nitriding process parameters and role of various alloying elements in the plasma nitriding process have been also discussed. If the steel has strong nitride forming elements (Cr, Al, Mo, etc.) under low concentration (~ 1-2%), the diffusion depth and hardness will be more. In the non-ferrous alloys, plasma nitrided samples have two or three distinct layers that depends on the plasma reactivity. Nitrided layer increases only until the critical time and temperature (4500C) were reached.
Nitriding is a thermo-chemical process. During this process, nitrogen is introduced into the surface of ferrous and nonferrous alloys as interstitial solid solution. Plasma Ion nitriding is an extension of conventional nitriding processes using plasma-discharge mechanism. The frequently used conventional nitriding processes are salt bath or liquid nitriding, gas nitriding, and chrome plating. The traditional salt bath nitriding was processed using liquid salt containing cyanide and cyanate which were toxic and increases ground pollution (Lee et al., 2010). However, later on air accelerated salt bath technique called 'Sursulf Nitriding (SN)' was developed in Germany, which is a non-toxic process (Murthy and Rao, 1983). In this process, samples were immersed into the molten salt bath, resulting in an uniform nitride layer formed at the surface of the sample.
During this process, some salt was deposited on the surface of the sample that must be needed proper cleaning after processing (Bacci et al., 2001). In the gas nitriding process, ammonia (NH3) gas was used for the treatment of sample surface. It is an effective process to improve the surface hardness and wear resistance of steel samples, but longer treatment time is required (Pessin et al., 2000). Further, a brittle nitride layer is formed and some dimensional changes occurred during the process (Bacci et al., 2001). The aforesaid difficulties have been removed in the plasma nitriding process (Ochoa et al., 2009). It is a diffusion process which can be carried out at low process temperature. It provides many other advantages over traditional nitriding process, such as reduced gas consumption, reduced energy consumption, and it is an environmental friendly process (Hannula et al., 1989; Collins et al., 1995; Marchev et al., 1998; Larisch et al., 1999; Musil et al., 2000; Borges et al., 2001).
Since the mid-1960s, plasma nitriding equipments have been commercially available (Mahboubi, 1998; Kopeliovich, 2012). Plasma nitriding technique was first used as a metallurgical processing tool (Skonieski et al., 2013). The plasma nitriding is also known as Glow Discharge Plasma Nitriding (GDPN) and Plasma Ion Nitriding (PIN). In the plasma ion nitriding process, electric current flows between two electrodes were placed in a sealed gas environment. The gas atoms become excited and are driven along a very short mean free path and collide with other atoms. During this process, energy is released and a glow is seen; the colour of glow depends on the type of gas used. The principle of glow discharge phenomenon is well understood by Paschen curve. In I-V characteristic of a DC discharge, different regions appear like: Townsend Discharge, Corona Discharge, Subnormal Glow Discharge, Normal Glow Discharge, Abnormal Glow Discharge, and Arc Discharge, respectively. Plasma ion nitriding is done in the abnormal glow region (Liang et al., 2001).
In this review paper, the solubility of nitrogen in iron and formation of stable and unstable compounds have been discussed through Fe-N system in Section 1. The different kinds of nitriding processes have been discussed in detail in Section 2. Plasma nitriding process is described separately in Section 3. Plasma nitriding experimental setup is described in Section 4. The findings of plasma nitriding process and effects of various process parameters on surface properties have been described in detail in Section 5. Conclusions of this study are given the last section.
Nitriding is a thermo-chemical process of diffusion of nitrogen atom in the surface of steel. Diffusion process is strongly dependent on the solubility of nitrogen in iron. An equilibrium phase diagram of iron-nitrogen (Fe-N) binary system has been shown in Figure 1. From the study of Fe-N system, it is seen that it consists of several solid solutions of N (α, γ, ε), stable chemical compounds (γ' Fe4N, ξ-Fe2N), and metastable phases (α' -martensite, α”-Fe16 N2 ). The solubility of atomic nitrogen (N) in the body centered cubic (bcc) lattice of γ' -Fe4N is about ~ 0.4 at %w N without noticeable changes in bcc lattice. In the phase, N can be dissolved to a maximum of 10.3 at %w. The ' γ' -Fe4 N phase has a narrow composition range of 19.3−20.0 at % N. In ε-FexN phase, N can be dissolved about 15 at % N to at %N. However, the solubility limit of nitrogen in iron is temperature dependant. At 450°C, iron absorbs nitrogen between 5.7 and 6.1 wt%. Beyond 6.1 wt% of nitrogen, the epsilon phase (ε) predominantly forms on the surface. This ε phase is strongly influenced by the carbon content present in the steel. Higher carbon content, required more potential to form the ε phase. As the temperature is further increased upto 490 °C, Fe4N ( γ’) phase is formed. The solubility of N decreases at temperature of approximately 680 °C. The equilibrium diagram shows that the control of the nitrogen diffusion is critical for success of plasma nitriding process (Pye, 2003; Kunze, 1990).

Figure 1. Iron-nitrogen Equilibrium Phase Diagram (Pye, 2003)
In the gas nitriding process, nitrogen is introduced into the surface of steel by holding it at a temperature between 495°C and 600 °C in a Ammonia (NH3) gas environment. The workpiece is heated in a furnace and ammonia gas begins to decompose. After certain reactions, atomic nitrogen forms and it diffuses into the steel surface. The typical thermo-dynamical reactions of decomposition take place at the steel surface in the following manner (Pye, 2003; Thomas et al., 1992; Spencer, 1992; Vandervell and Waugh, 1990; John, 2005):
NH3 ---------- 3H + N
2N------------- N2(2)
2H--------------H2(3)
At the instant of decomposition, the atomic nitrogen can be adsorbed physically or chemically at the steel surface. The physically adsorbed atomic nitrogen and hydrogen are unstable and recombines to form nitrogen and hydrogen molecule. While, chemically adsorbed nitrogen atom have higher probability of getting diffuse in iron and steel at this elevated temperature. Higher temperature increases the probability of nitrogen diffusion faster into the deeper depth. The atomic nitrogen has diameter 1.42 Å and is dissolved in iron in interstitial position in octahedral voids of cubic lattice. These voids have the diameter of 0.38 Å in bcc iron and 1.04 Å in fcc iron. The process of diffusion of nitrogen at suitable process temperature is known as interstitial diffusion.
Pack nitriding is also known as powder nitriding. In this process, the samples are packed in powdery solid nitrogen bearing compounds in the presence of water, e.g. CaCN2 , with an addition of clay minerals. They are heated up to nitriding temperature (470-570 °C) in a furnace. At this nitriding temperature, compound slowly decomposes and provides necessary nitrogen for the nitriding treatment as given in equations below.
CaCN2 + 3H2O→ CaO + CO2 + 2NH3C + CO→ 2CO
CO2 + H2→CO+H2O
2NH3→2N+3H2
In ion implantation process, the ions of one material is implanted into another solid material, by which the physical and chemical properties of the solid materials are changed. This change in the physical property (structural change) produces a surface compression in the material. The produced surface compression prevents crack propagation and thus makes the material more resistant to fracture (Pulkkinen and Lähdeniemi, 1984; Conard, 1987; Tendys et al., 1988; Conard et al., 1987). The chemical changes in the material also improve corrosion resistant property. In the complete process, an ion beam of the energy range between 40 keV and 1 MeV is used. This ion beam penetrates into the iron surface to form an iron nitride layer at low temperature (Terwagne et al., 1989a, 1989b; Jagielski et al., 1994).
In the ion implantation equipment, an accelerator is used to accelerate the ions at a high energy. A target material is put in the chamber on which ions are to be implanted. Each ion is typically a singly ionized atom, and thus the actual amount of material implanted in the target is the integral over time of the ion current. The current supplied by conventional implanters are typically small (microamperes), and thus the dose that can be implanted in a reasonable amount of time is small. Thus, ion implantation finds application in cases where the amount of chemical changes required is small. Typically ion energy in the range of 10 to 500 KeV are extensively used for implantation purposes. Energies in the range 1 to 10 KeV can be used, but result in a penetration of only a few nanometers or less. The typical depth of penetration of N on Fe is 1 nm/ KeV in the range of 1-100 KeV. Energies lower than this value resulted in very little damage to the target, and fall under the designation ion beam deposition.
In the laser nitriding of iron, high-power density lasers are used. In this process, iron nitride layer is formed with thickness in the micron and mean nitrogen concentration exceeding 10 wt%. However, the mechanism of the laser nitriding is still not clearly understood because of the various complicated interaction between the incident laser radiation with the molten material and atmospheric nitrogen takes place at the material surface (D'Anna et al., 1992; Barnikel et al., 1997; Han, 2001).
Liquid nitriding is also known as salt bath nitriding. In this process, a molten salt bath containing mixture of cyanides and cyanates are used as sources of nitrogen. The three process parameters, such as bath temperature, treatment time, and the composition of the salt bath determine the nitriding results. The typical treatment temperature in this technique is in the range of 550 to 580 °C. The main advantage of this process is short treatment times ~ 1 to 10 hours for nitrogen uptake (Lee et al., 2010).
In PSII, the ions are accelerated through a surfaceconformal sheath. Thus targets with larger area can be easily treated. In PSII, the substrate is immersed in low-pressure (10-2 -10-4 mbar) plasma with negatively biased power supply pulsed between -20 and -250 kV. The ion energy and dose was determined by surface bias and plasma conditions that may also be responsible to increase the surface temperature. The pressure and plasma density in PSII is very low in comparison to the glow discharge plasma nitriding. Since large negative bias is applied on the substrate, in initial phase ions remain stationary (for times less than ion plasma period defined as fpl = 2.1 x 102 zμni1/2 Hz), and electrons are repelled by the pi i bias voltage. During this phenomenon, an ion matrix sheath is created around the substrate (Gupta, 2011). For longer time scale, ions move towards the substrate and gain sufficiently high energy required for implantation. Under the typical PSII conditions, nitriding can be done with pure nitrogen plasma and hydrogen is not essentially required. PSII assures dose uniformity for the samples of complex shapes. However, the penetration depths are determined by the kinetic energy of the ions that is ~ 1 nm / + k eV for N+ on Fe. For higher depths, either the kinetic energy has to be increased or the implanted ions have to diffuse inside the substrate because of the surface temperature.
Shallow Implantation and Diffusion Hardening (SIDH) process incorporates diffusion of nitrogen in low pressure nitrogen plasma. In SIDH, the substrate is negatively pulsed biased of the ~ -1 kV to -20 kV. The repetition rate of the pulse and pulse duration depends on the required substrate for diffusion of implanted ions (Baldwin et al., 1997; Mukherjee, 2002).
Solution nitriding is a controlled heat treatment process for case hardening of stainless steels. This process is carried out at 1100 ± 50 °C in N2 gas atmosphere (Berns and Siebert, 1994). It uses the capability of stainless steel to dissolve in nitrogen at temperature above 1000 °C to a large extent without forming chromium nitride. A region of ~2 mm hard martensitic or a ductile austenitic phase with higher nitrogen percentage is formed. This region contributes to reduce wear by sliding, fretting, erosion, and cavitations. (Berns and Siebert, 1994; Siebert, 1994; Berns, 1994).
The plasma nitriding is also known as Ion Nitriding and Glow Discharge Plasma Ion Nitriding (GDPIN). In plasma nitriding, nitrogen-hydrogen plasma supplies ions, excited atoms and molecules as well as radicals for nitrogen incorporation into iron and its alloys (Pye, 2003; John, 2005; Lieberman and Lichtenberg, 2005; Williams and Poat, 1984; Moller and Mukherjee, 2002; Blawert, 2000). The typical voltage-current characteristic of a DC glow discharge mechanism is shown in Figure 2. During the phenomenon of gas discharge, various regions are formed; the two important regions, normal glow and abnormal glow are discussed below.
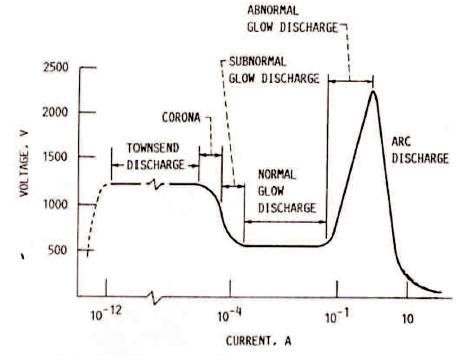
Figure 2. Voltage-current Characteristics of Discharge (Lieberman and Lichtenberg, 2005)
In this region, electron energy and number density are high enough to produce just enough ions, and a steady state condition is reached. In this condition, equilibrium is established between the rate of formation of ions and the rate of their recombination. At this stage, the discharge is self-sustaining and gas begins to glow. Such type of region is called normal glow region, in which the voltage drops and current rises abruptly and it is almost independent of the voltage. The minimum threshold voltage required to produce the glow discharge is called the breakdown voltage Vb.
At low power, the sustained glow discharge covers only the area near the rim of the cathode. When power is raised, the current increases and the discharge spread to cover the whole surface of the cathode uniformly. Such type of discharge is called abnormal glow discharge and it is very much useful in the processing of materials. In the abnormal glow discharge, current can be easily controlled. Thus, the plasma nitriding is generally done in abnormal glow region because the ideal conditions exist. Typically, the substrate is biased negatively between 400 V to 800 V having current density 1-5 mA/cm2 (Lieberman and Lichtenberg, 2005). The typical existing plasma ion nitriding mechanism with formation of iron-nitrogen compounds are described in Figure 3.
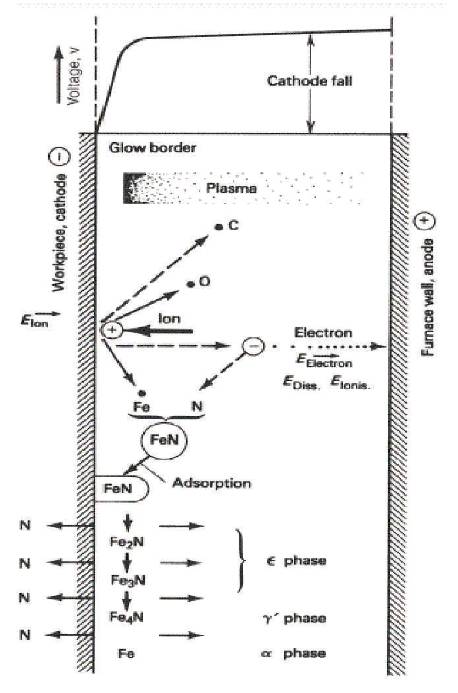
Figure 3. Glow Discharge Ion Nitriding Mechanism (Pye, 2003)
Plasma nitriding is a metallurgically versatile technique which provides excellent dimensional control and surface finish. Plasma nitriding can suppress the formation of whitelayer and if needed can form a monophase layer, which may be either epsilon (ε) or gama prime (γ') in nature. In the plasma nitriding process, repetitive metallurgical results can be produced and also the complete process can be controlled to achieve desired nitrided layers. Moreover, the process control ensures high dimensional stability, eliminates secondary operations, offers low operatingtemperature capability, and produces parts that retain surface finish. Apart from the operational control, it has also the following technical and social benefits, such as environmental friendly, selective nitriding by simple masking techniques, reduced nitriding time, minimal gas consumption and ability to treat almost any steel, etc. Every process has its own limitations. The limitations of plasma nitriding include high capital cost and requirement of precision power supply.
The schematic diagram of experimental set-up for plasma ion nitriding is shown in Figure 4. The main components of the experimental setup are, Vacuum Pumps, Gas Cylinders, Gas Mixing Chamber (GMC), Mass Flow Controllers (MFC), Pulsed DC Power Supply, Thermocouple, and necessary arrangement of auxiliary heaters.
Initially, the chamber is evacuated upto the base pressure of ~5x10-3 mbar with the help of a rotary and a root pump. After that, Argon and Hydrogen gases are introduced into the chamber in a pressure ratio of 1:4 to maintain a gas pressure of 1 mbar. The gas pressure is measured by a Baratron gauge. At this gas pressure, initially a glow discharge plasma is ignited between chamber wall as anode and substrate table as cathode by applying pulsed DC voltage of ~400 Volts at ~30 kHz frequency. This Ar-H2 discharge is utilized to remove the contaminants from the sample surface as a cleaning process. After an hour, N2 -H2 gases are introduced into the chamber in a pressure ratio of 80:20 through Mass Flow Controllers (MFC) and Gas Mixing Chamber (GMC). A typical working pressure of ~1 to 10 mbar is maintained for carrying out the plasma nitriding process. A thermocouple is used to measure the sample temperature. The plasma nitriding operating parameters are controlled and monitored by a PLC controlled computer.
Figure 4. Plasma Nitriding Experimental Setup (Kumar et al., 2016)
In the plasma nitriding process, various process parameters are involved. These process parameters can affect utility of the PIN process. Many researchers have worked and explored the effect of various process parameters. Some of these are reviewed under various sections.
In PIN process, nitrogen and hydrogen gases are used. Nitrogen gas is responsible to form nitride, but the role of hydrogen in the nitriding process is not clearly understood. Many research papers show that it enhances the discharge current and increases the diffusion rate of nitrogen and behaves as a catalyst (Pye, 2003). Bougdira et al. (1991) studied the effect of hydrogen in a pulsed plasma nitriding. They found that the presence of a small amount of H2 in the gaseous atmosphere (less than 10%), increases the excitation of iron atoms and of nitrogen molecules. Moskalioviene and Galdikas, (2015) have proposed a numerical model, to analyze and describe the effect of hydrogen on plasma nitriding of austenitic stainless steel. According to this model, when hydrogen was added in the range ~(30 to 40) pct, nitrogen penetration in steel was increased. It also reduces the surface oxide and increases the NH radicals, which are converted into active nitrogen atoms on the steel surface, i.e., the amount of adsorbed and diffused nitrogen increases as compared to pure nitrogen plasma. Borges et al. (2000) studied the effect of hydrogen on SS 304 during plasma nitriding. They observed that a high concentration of hydrogen decreases the chromium concentration on the surface of steel. Sharma et al. (2006) studied the effect of hydrogen during pulsed DC plasma of AISI 302/304 austenitic stainless steels. They reported that in pure nitrogen plasma, surface hardness did not increase from the initial value. In N2 –H2 gas plasma, surface hardness increases ~3-4 after plasma nitriding. Kumar et al. (2011) studied the effect of gas (N2 –H2 ) compositions on plasma nitriding of AISI 52100 ball bearing steel. They reported that surface hardness has increased to ∼ 585 HV from its initial value (262 HV) when samples were plasma-nitrided with 95% nitrogen and 5% hydrogen gas as compared to plasma-nitriding with 65% nitrogen - 35% hydrogen and 25% nitrogen - 75% hydrogen gas. On the basis of these different observations of the authors, it can be concluded that hydrogen plays an important role in the plasma nitriding process. In the presence of hydrogen gas, diffusion process of nitrogen in steel matrix increases due to increase in surface current density that increases the presence of active nitrogen on steel surface for diffusion.
Petitjean and Ricard (1984) made an Emission spectroscopy study of N2 -H2 glow discharge plasma. In this study, N2 , N2+ , N, N+ , H, NH, and metal atom excited states have been detected. In another spectroscopic study, Guillermet and Hong (1994) show that N2+ , N+ , NH, Hα , and Hβ species exist in the nitriding process, but nitrogen ions (N+) were the most dominant species. Nitrogen to hydrogen gas ratio depending on the requirement of surface metallurgy, fixed gas chemistry gives the fixed surface metallurgy and variable gas chemistry allows a variable surface metallurgy (Pye, 2003). Spencer (1992) studied the surface metallurgy of the plasma nitrided component and it depends on the process parameters like: partial pressure in the chamber, applied voltage, surface current density, surface temperature, and process time duration. A detailed study after plasma nitriding of AISI 5140 steel with various gas mixtures has been reported by Vandervell and Waugh, (1990) and Pulkkinen and Lähdeniemi (1983).
Effect of the gas pressure on the excited species during plasma nitriding process and on the microstructural changes observed were studied by Jeong and Kim (2001). In this study, it was observed that the emission intensity of N2+ and N2 increases with increasing gas pressure even if the current density was kept constant. The intensity ratio of these species was also increased with increasing gas pressure. This study leads to an important conclusion that N2+ was a more decisive active species than N2 and has an effect on the formation of the nitride layer. The behavior of nitrided layer was studied on high speed steel at different gas pressures. It is found that the thickness of the compound layer and diffusion zone increases with increasing gas pressure. An explanation of this behavior was given by Takahashi (1993). According to Takahashi (1993), the mean free path of plasma species becomes shorter when gas pressure was increased, due to this fact kinetic energy of charged particles were reduced. This may result in a decrease of ion energy and ion bombardment population by discharged ion and molecular particles. Therefore, deposition effects may be dominant compared to sputtering. A detailed study of the effect of various gas mixtures on AISI 5140 steel was reported by Karakan et al. (2002). In this study it was found that the surface hardness and diffusion depth are maximum for the gas ratio of 10% N2 and 90% H2 .
Díaz-Guillén et al. (2009) studied the effect of duty cycle on the surface properties, such as surface hardness, diffusion depth, compounds’ zone width, and crystalline phase composition for AISI 4340 steel. In this study it has been found that the treated samples show better result when duty cycle was kept ~50% - 70%. Alphonsa et al. (2008) studied the effect of frequency of the applied pulse DC source on the micro-structure and corrosion resistance of AISI 4340 steel at 10 kHz and 30 kHz. In this study, it was found that plasma nitriding with 30 kHz frequency gives better corrosion resistance and higher surface hardness than 10 kHz on AISI 4340 steel. Taherkhani and Mahboubi (2013) studied the combined effect of source frequency and duty cycle on surface properties of AISI H13 steel. They observed that with increasing frequency and duty cycle, thickness of the compound zone, surface roughness, and surface hardness were increased. Varman and Huchel (2017) studied the effect of pulse repetition time on surface properties of plasma nitrided AISI 4340 Steel. They reported that strong iron nitrides of gamma and epsilon were observed at reduced pulse repetition and disappeared gradually when pulse repetition time was increased. The compound layer thickness was found maximum when pulse repetition time is zero (PR-0). On the other hand, wear resistance was higher for the samples of PR- 300 due to the presence of Chromium Nitrides. Naeem et al. (2017) studied the effect of duty cycle on tribological and corrosion properties of AISI 316 stainless steel. The results of this study show that hardness was increased at low duty cycle. The wear rate was found to be reduced up to 90% and corrosion rate was found to be reduced up to 95% when material was processed at low pulsed duty cycle (15%) in cathodic cage plasma nitriding. On the basis of above literature review, except the observations of Naeem et al. (2017), higher duty cycle was helpful to increase the surface hardness, thickness of compound zone, and wear resistance properties of nitrided material.
Mendes et al. (2014) studied the effect of plasma nitriding treatment time and temperature on kinetic layer growth on stainless steel and nickel steel. The results of this study reveal that the nitrided layer thickness increases for higher treatment temperatures. At lower nitriding temperature (350 oC – 380 oC), expanded austenite phase were formed. At this temperature range, chromium-nitride (CrN) peak was not detected, but at higher nitriding temperature, CrN peak was detected under XRD investigation. Formation of chromium nitride in stainless steel looses its corrosion resistance properties. Surface hardness was found to increase with increasing process temperature. On the other hand, surface hardness was found to increase with increasing process time, but for longer process time, it will be decreased. Sirin et al. (2008) studied the effect of the ion nitriding on AISI 4340 steel. In this paper, they reported that surface hardness and case depth of AISI 4340 steel was increased by increasing plasma nitriding time and temperature. It is also found that the maximum hardness and the maximum case depth do not coincide. At higher treatment time surface hardness decreases while the case depth was found to increase continuously. Wang et al. (2006) studied the effect of plasma nitriding time on AISI 304 austenitic stainless steel. They reported that thickness of the nitrided layer and surface micro-hardness were increased with nitriding time. The value of micro-hardness was not increased for increasing the nitriding times more than 7 h at constant temperature. X-ray diffraction study reveals that the expanded austenite (γN) phase were present in the nitrided layer when samples were treated at 420 oC. Ogale et al. (1987) and D'Anna et al. (1992) studied the plasma nitriding process to increase the mechanical properties like: surface hardness, wear resistance and fatigue strength of steel components. Barnikel et al. (1997) studied that plasma nitriding process also increases the chemical property like corrosion resistance, except stainless steel. The thermal fatigue behavior, wear behavior, and hardness depth profile study have been thoroughly reported by many researchers (D'Anna et al., 1992; Barnikel et al., 1997; Han, 2001). The microstructure study, phase study, and residual stress measurement study of many steel samples after plasma nitriding have been made by Conard (1987), Tendys et al. (1988), and Conard et al. (1987). Mechanical properties of nitrided steel were influenced by processing parameters (Berns, 1994; Ogale et al., 1987). On the basis of literature review, it was observed that the mechanical properties were increased after plasma nitriding. Some properties such as case depth and surface hardness shows reverse trend at higher treatment time.
Many researchers (Egawa et al., 2010; Gouné et al., 2000) studied the role of alloying elements for improving the surface properties after plasma nitriding process. Egawa et al. (2010) suggested that the presence of strong nitride forming elements enhances the nitrogen diffusion depth in austenitic stainless steels. Gouné et al.’s (2000) observations support the role of Cr for increasing the diffusion of nitrogen in the bulk of the material. Additionally, Wang et al. (2006) explored, in detail with the help of computational model, the presence of Cr% to limit the diffusion depth in plasma nitriding. This study importantly reveals that the diffusion depth of nitrogen increases with increase in Cr% till 1% while it decreases with more Cr%. Hossain et al. (n.d) explored the role of unstable nitride forming element Nickel (Ni). The surface micro-hardness of Ni containing low carbon, Cr steels reduces after plasma nitriding process. Ni containing steels have more retained austenite that reduces the surface micro-hardness. During the nitriding of Ni containing austenitic steels, coarsening of nitride segregation in the nitrided layer may be another reason for reducing the surface micro-hardness. Kumar et al. (2016) studied the role of alloying elements on surface properties of various alloys. This study reveals that variation of micro-hardness (H) in diffusion depth (X) follows a power law HαX-2 . It was observed that alloying elements and their concentrations available in the steel sample play a significant role. They conclude that if the steel has strong nitride forming elements (Cr, Al, Mo, etc.) under low concentration (~ 1-2%), the diffusion depth and hardness will be more.
Apart from the detailed study on various ferrous alloys, nonferrous alloys were also investigated. Borowski et al. (2009) studied the modifying properties of Inconel 625 Nickel alloy. It was concluded that the diffusion-type chromium nitride layer produced on the alloy surface improves the hardness, frictional wear resistance, fatigue strength, and corrosion resistance of the alloy. Dahm et al. (2007) characterized the plasma immersion ion implantation (PI3) of a commercial Ni-base alloy (Inconel 601). All of the nitrided samples showed superior corrosion resistance to the untreated alloy in 3.56 wt.% NaCl. Leroy et al. (2001) studied the Inconel 690 alloy. It was found that low temperature plasma nitriding improves the tribological properties without changing the corrosion resistance. The plasma nitrided samples have two or three distinct layers that depends on the plasma reactivity. These layers were related to the three different metastable f.c.c. nitrogen solid solutions. Mindivan and Mindivan (2013) studied the wear performance of hardened Inconel 600 by different nitriding processes. It was concluded that wear rate was lower for the pulsed plasma nitrided samples, but the nitriding processes did not significantly affect the surface hardness of such alloy. Sudha et al. (2013) studied the nitriding kinetics of inconel 600 alloy. In this study, it was found that surface hardness increased from 200 to 1260 VHN (at 600 °C for 24 h). Growth kinetics was followed by a parabolic rate equation and was controlled by the diffusion of nitrogen. The calculated value of activation energy for diffusion was obtained as 0.65 eV, which is close to the apparent activation energy for diffusion of nitrogen in a Ni matrix. Kahraman and Karadeniz (2011) studied the wear behavior of plasma nitrided nickel based dental alloy. It was concluded that different nitrided layers were formed during nitriding process. It was also concluded that the nitrided layer increases only until the critical time and temperature (450 °C) were reached. Above these critical values, it was observed that the layer thickness decreased. The maximum wear resistance was observed at 400 °C for 10 h due to the high hardness and thickness of the nitrided layers.
On the basis of this critical review, the following conclusions have been drawn.