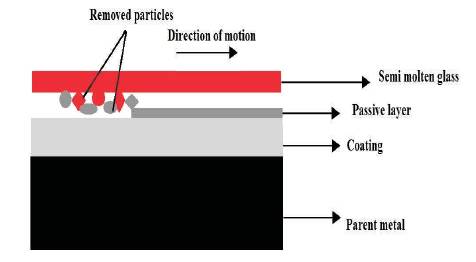
Figure 1. Schematic Diagram of Three Body Abrasion on Interface of Molten Glass and Die Surface
This paper reviews all coating techniques, including Chemical Vapour Deposition (CVD), Physical Vapour Deposition (PVD), Magnetron Sputtering, and Ion Beam Assisted Deposition used for the coating on the working surface of dies used in glass manufacturing industry. Coatings used with these techniques were single layer coatings of nitrides, borides, and oxides, multilayer coatings of nitride or carbides and noble metal like Platinum-Iridium (Pt-Ir) coatings. During glass manufacturing, dies are subjected to high temperature ranges from 900 °C to 1150 °C. The characteristics of coatings like wettability, wear and corrosion resistance against thermal cycling at high temperature are important factors for die manufacturing materials. Problems related to these characteristics have been elaborated in detail which reflects the behaviour of coatings particularly used for dies, and their effects have been studied. Oxide scale formation on the working surface and coatings due to high temperature corrosion is also a burning issue and it has been explained in this review paper. Among the various coatings, Pt-Ir coatings are the best in terms of performance. However, these noble metals are costlier than other materials which have been used for coatings. Further the different coatings for high temperature corrosion applications have been taken into consideration to know the effective results associated with their use and the materials that have provided the protection to the working surfaces at high temperatures. It has been found that the coating process, its parameters and selection of material directly affect the surface properties at high temperatures and it is proposed that the use of new coating techniques, such as microwave cladding and thermal spray coating with a number of variants in their processes and feedstocks can effectively modify the surface properties of die materials.
In actual practice, 90% of engineering problems are started from the working surfaces of engineering components from corrosion, erosion and wear. To mitigate these problems the surface properties of the engineering components can be changed by using suitable techniques, such as coating, cladding, and glazing. The developed coatings must exhibit properties, such as high wear resistance, corrosion resistance, anti-sticking behaviour at high temperatures, and higher thermal conductivity. Protective coatings provide up to 10 to 50 times increase in tool life (Goswami, 2004).
There is need to develop such protective surface which not only prevents degradation but also serves for long time. Tribo-corrosion is a very critical area in surface engineering where surface is exposed to loss of material by mechanical action along with chemical action in the form of corrosion and it is quite common where material has to withstand high temperatures. It includes plethora of wear mechanisms for failure of material depending on the material properties and type of action. For instance, in case of glass manufacturing dies the sticking occurs due to adhesive wear on the interface of molten glass and die surface. Abrasive wear also occurs at the interface of materials which are in contact during the relative motion. This paper presents the application of die that is used in the glass manufacturing industry. In this case, the molten glass rub against the die surface at high temperature more than 500 °C as the transition temperature of glass is 570 °C. The particles from die and glass surface are detached due to this mechanical action and sticks on the glass surface whereas grooves formed on the surface due to ploughing also form scratches as well. Figure 1 shows the three body abrasive wear along with removal of passive layer at the interface of coating and molten glass which is in relative motion with each other. Oxide scales are loosely bonded to the surface and they also get detached by the mechanical action.

Figure 1. Schematic Diagram of Three Body Abrasion on Interface of Molten Glass and Die Surface
Coatings are solutions for the protection of working surfaces, however in case of motion and high temperature it becomes very difficult to protect the surface as the surface energy of the material changes with increase in temperature and selection of suitable material is also a main aspect. Figure 1 clearly shows the passivating layer developed on the coating which is being removed due to the motion and particles present at an interface. The performance of the die materials has an important role in glass manufacturing. Process parameters and properties such as temperature variation in moulds due to thermal cycling operation, surface finish of the mould and most importantly life of the glass mould material have been discussed by many researchers (Nishiyama et al., 1996). There have been reports of coatings on glass moulds with different materials to enhance the life of the mould. Preferred glass mould materials, such as tungsten carbide, stainless steel, silicon carbide, titanium nitride have been tested (Brand et al., 2004) and grey cast iron grade is the commonly used material for glass moulds and due to high hardness of this material, the process of fabrication and repair becomes difficult and costly (Yan, J. et al., 2009).
Corrosion and wear both occur in the glass forming process which may lead to pitting on the surface of die and hence produces surface roughness whereas corrosion occurs along the grain boundaries, due to which material of the mould sticks to the glass surface and such process may decrease the quality of the glass, and oxidation due to the cyclic operation on cast iron at 500-650 °C reduces its strength characteristics. Previous researchers have also shown the role of fracture toughness on mould materials. Toughness is directly linked with gob which is basically a piece of molten glass, when entered through the edges of die produces an impact wear and is pressed by the plunger and to withstand against this condition die material requires toughness (Nishiyama et al., 1996).
Glass is a transparent material and non-crystalline substance. Sand (SiO2), Sodium Carbonate (Na2 CO3), and Limestone (CaCO3) are the main ingredients used as a raw material for the manufacturing of glass. These chemicals lower the melting point of sand, main constituent of raw material and softens the glass for easy melting. Raw material is then mixed with cullet, which is basically a recycled glass. Cullet helps in lowering the melting point of the raw material. After that whole mixture is heated in the furnace at 1000 °C.
The following chemical reaction takes place and gives glass in a liquid state:
Na2CO3 +CaCO3 +4 SiO2 → Na2SiO3.CaSiO3. 4 SiO2 +2CO2
Impurities called Glass Gall rise to the surface and is removed. The liquid glass is poured into moulds to get the required shape (Ma, et al., 2008). Figure 2 shows the main steps in the manufacturing of glass.
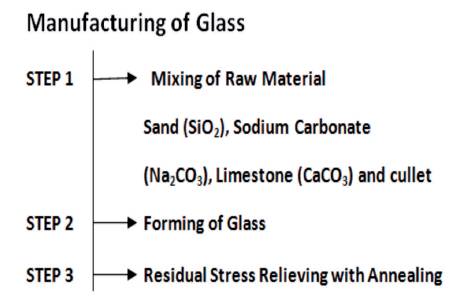
Figure 2. Steps in Manufacturing of Glass Making
Glass obtained by rapid cooling on releasing from moulds is brittle. When new glass is formed, the outside surface cools rapidly than inside surface causing stresses (residual or body stresses) in the glass. To relieve these stresses induced in glass, annealing is used, which strengthens the glass. Molten glass is cut by blade called shears. These shears cut elongated cylinders of glass which are termed as gobs. Each gob has correct amount of glass to produce one container and bottle (Cable, 1999). At this stage, they are ready to be formed into glass container and delivered to the forming machine. Figure 3(a) illustrates forming machine which uses compressed air to transform gobs into containers and bottles in two steps process. Figure 3(b) shows, gob is delivered to blank side of the machine where it is formed into parison, which is a blank form of container. It is then transferred on to the mould side of the machine, where it is blown into the final shape of the container.
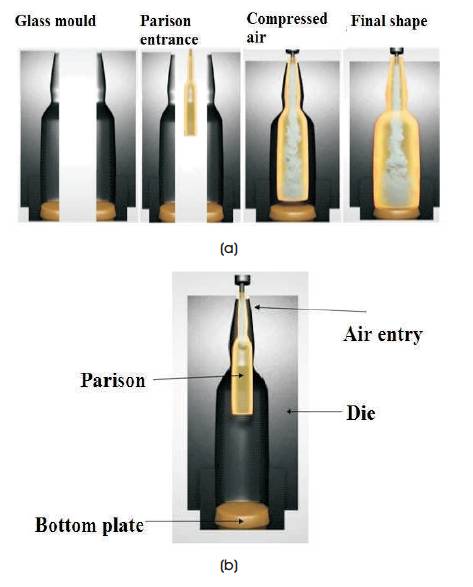
Figure 3. (a) Illustration of Glass Forming Process (b) Press and Blow Process
This paper is mainly concentrated on the second step of manufacturing of the glass, i.e. forming of glass in the moulds. In the selection of coating for glass manufacturing tools, such as mould, plunger, neck ring, bottom plate, and guide ring, researchers have faced many challenges, such as component geometry as the plunger is cylindrical, the design requirements, operating temperatures, die materials, determination of the process, and coatings to be used.
Sticking is a very critical problem in the case of high temperature applications. Some elements which are present in die material losses their retaining properties at high temperatures which further affects the other elements and finally leads to detachment of the material from the surface. Wettability is directly related to the contact of liquid with the material which is having solid state (Rieser et al., 2008; Zhong et al., 2000). The spreading of material on the solid surface leads to sticking which is a well known failure in tribology, known as adhesive wear. This causes the localized welding of the material at high temperature and on cooling the softer material gets detached from the surface. This mechanism has been found in case of dies where cooling system is used for the ejection of the components from the dies. Working surface of die in contact with molten glass is showing different wetting angles by a schematic representation given in Figure 4.
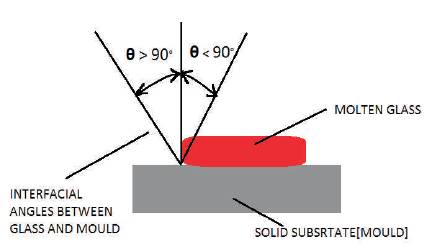
Figure 4. Schematic Diagram of Die Surface Contact with Molten Glass showing Different Wetting Angles
Contact angle is the measure of the wettability of the material which is in contact. If the contact angle is less than 90° or it is between 0° to 90°, then it is a favourable wetting condition while on the contrary, angle between 90° to 180° is known as non-wetting condition. The stability of the material depends on the interfacial surface energy per unit area and this is an important factor to consider for the material selection for the die coatings. The material which is having low surface energy at high temperature will retain their properties and do not wet by the hot component. This type of stable materials include some oxides and nitrides like Al2O3 and some elements like nickel, cobalt, and boron (Callister et al., 2017). Therefore stability of oxides and elements at high temperature is required to avoid the hostile condition of wettablility.
Moreover, the adhesion at the interface of die and glass occur due to two main reasons. First and foremost reason is the adhesion of molten glass, the which sticks to the die material due to high temperature and secondly the low temperature leads to damage of glass due to cooling. These both conditions have adverse effect on the glass surface due to its brittle nature. Adhesion of coatings to the base metal has always been a problem due to improper selection of coating materials (Masuda et al., 2011). Molten glass has a normal temperature range between 900 °C to 1150 °C and temperature of die is held below 500 °C. Both sides of the glass have contact with the metal, i.e. the interior of the glass with plunger and at the same time, exterior with mould. Due to high temperature at interfaces, the conduction and radiation both occur during heat exchange between these interfaces (Kleer and Doell, 1997; Viskanta and Lim, 2001) which accelerates the formation of oxide scale.
In addition to adhesion at high temperatures, the dies also face a problem of abrasive wear at the interface of materials. In case of glass manufacturing dies there are two interfaces at which this problem occurs. First is the contact surface between molten glass and die surface and second is the interface of plunger and molten glass. The production rate of glass manufacturing is very fast and motion of the contact surfaces are purely reciprocating and therefore the material is lost due to high rate of reciprocating abrasive wear. The strength of the material is lost due to this mechanical action and microcracks are formed on the surface of the glass which reduces the strength of the glass and also deteriorates its surface. This directly indicates the wear mechanism of ploughing that is the formation of pits on the surface of the material. It is better to use high performance alloys and combinations of elements to curb the problem of abrasive at an interface (Stliner, 1990).
This is basically because of the formation of oxides at high temperature mainly due to presence of oxygen. The surface of the material gets damaged due to oxidation. Hones et al., (2000) have reported that due to fast production rates the mould require immediate cooling less than 20 seconds and that causes thermal cycling operation in dies. Corrosion removes the large particles from the grain boundaries and these particles take place at the surface of the component and hence decrease the surface quality of the component. Grain boundaries act as an obstacle to the dislocation which prevents the material to be plastically deformed (Callister et al., 2017). It is reported that nickel based alloys have such properties that mitigate the problem of oxidation at high temperature by forming such phases which provides protective coating on the surface of the material (Kalss, W. et al., 2006). The structure of these alloys remains dense even at higher temperatures. The stability of these elements depends on the free surface energy of the element and the shape of the particles of the material to be used for the coating. It has been reported in the study of coatings by thermal spraying process and microwave cladding that the bonding of the material in the form of powder particles has a dependency on the size and shape of particles. The spherical particles of the powders have lowest surface energy due to low volume to area ratio whereas the use of nano particles for the coating of a surface provides more strengthening to the coating. This is due to the fact that as the size of the grains decreases the volume of the grain boundaries increases which acts as a barrier for dislocations.
For oxidation study of stainless steel as a substrate, it was initially surface boronized and then tested in thermal cycling conditions on laboratory setup and its surface roughness was measured and reported that the surface roughness increases with increase in number of thermal cycles (Akdogan and Durakbasa, 2008). It is obvious that with passage of time, the surface gets oxidized which ultimately ceases with failure of oxidation due to spalling and oxide scale formation on the working surface of the die.
In addition to coatings, alloying of elements with cast iron played a significant role in the manufacturing of moulds. The tensile strength and wear resistance of cast iron decreases at high temperatures (Kim et al., 2009). However, the casting is followed by heat treatment processes to decrease the residual stresses, but these processes lead to structural transformation in cast iron and formation of graphite inclusions (Leushin and Chistyakov, 2014). Alloying elements, such as molybdenum, nickel, and chromium when added to cast iron, enhance the wear and corrosion resistance at high temperatures (Hemanth, 2000). It has been found that alloying of nickel, molybdenum, and copper with grey cast iron enhances the wear and oxidation up to 20% as compared to unalloyed grey cast iron (Cingi et al., 2002).
Cast iron is a hard material, but this is not only the factor to be considered in case of glass moulds. Alloying with elements like nickel increases the thermal resistance of die material. Different alloyed cast irons were tested for wear resistance, which showed good wear resistance at 600 °C and it lost their resistance at 800 °C (Mashloosh, 2015).
However, oxidation effects are minimized by introducing nitrogen in press chamber (Weck et al., 2003). Due to higher thermal conductivity, aluminium and bronze are the materials used for glass moulds but at present it costs twice the cast iron. Oxide layer has been formed on the working surface of the glass mould made of cast iron (Cable, 1999).
This process involves the splat by splat deposition of powder with high temperature and velocity on the substrate. This process has number of variants which are High velocity oxyfuel, Detonation gun spray, and plasma spraying. These processes can easily provide very hard, dense and smooth coatings with accuracy and these processes are used for high precision components like aeronautical components, steam turbine blades and dies (Holmberg and Mathews, 2009). The schematic illustration of this process is shown in Figure 5.
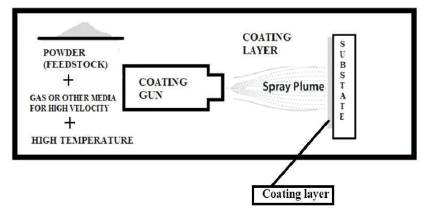
Figure 5. Schematic Representation of Thermal Spray Coating Process
TiO2-13Al2O3 coatings have been used for resistance against wear corrosion with plasma spraying technique. In this process, the powder is sprayed in the form of liquid droplets on the substrate and this finally plastically deformed after condensation. These coatings provide higher bond strength. Plasma spraying technique is used for ceramic coating due to high temperature which completely melts the powder particles and high velocity prevents the decomposition of powder particles and they do not interact with the oxygen and unwanted phases are avoided (Priyantha et al., 2003). Composite coatings provide some phases which prevents the surface of the die (Hock et al., 2003). Zirconia coatings with plasma spraying technique have been used on gas engine components for increasing their strength at high temperature (Liang and Ding, 2005). Zirconia is a light element and it goes into the grain boundaries and makes the structure denser. Grain boundaries are basically open structured and when zirconia diffuses it provides barrier for the dislocations to move. It also has a great role in Ni3Al single phase when added to it provides strength. Ni3Al single phase not only provides strength to the coating, but also decreases the corrosion rate. For this purpose, Ni3Al plasma sprayed coatings were used on boiler steels. This coating showed very effective results against high temperature corrosion (Sidhu and Prakash, 2003). Apart from zirconia there are some other light elements like carbon and boron that can be used with Ni3Al single phase coating to increase its strength. The actual environment conditions in case of boilers in thermal power plants are not very different from die used for glass manufacturing. In thermal power plants, steam is generated through boilers and that is obtained by heating of water with the help of coal. Heating of coal produces ash particles known as soot and it is in the form of black powder and it contains similar elements which are present in glass materials like silica, alumina, and magnesia, and this further contains carbon and oxygen to form gases which rapidly damages the surface of heat exchanger walls, soot blowers regions and the boiler surface of heating zone surface area. Presence of these constituents accelerate the corrosion rate and results in premature failure of surface of components. Ni-20Cr coating has been applied with high velocity oxy-fuel technique on boiler steels and these showed resistance to hot corrosion under thermal cycling actual environment of thermal power plant (Sidhu et al., 2006).
Nanostructured coatings have been used and they significantly enhance the properties like porosity by making dense structures which prevent the surface from corrosion. Nanomaterials especially for metals are referred to powders having average particle size below 100 nm. The decrease in their size below 100 nm enhances their strength and surface properties and this is due to ratio of grain boundaries volume to total area. Thickness of grain boundaries gets increased with decrease in particle size which acts as barrier for dislocations (Zafar and Sharma, 2016).
Nanocrystalline powders of Al2O3-13TiO2 were sprayed with plasma spraying and then compared with micron sized powder and this has showed better wear resistance than conventional powder (Bansal et al., 2003). Another study of nanocrystalline powder of Al2O3-13TiO2 include its performance in high temperature corrosion where it performed effectively up to 600 °C (Lin et al., 2004).
The study of high temperature coatings reveals that the nickel based coatings have great influence on the performance of the working surface. Conventional powders having average particle size of 74 microns and nano powders having particle size of 67 nm were deposited by thermal spray coating technique called cold gun spray method and their results have shown higher resistance to oxidation and increase in hardness as well (Kaur et al., 2015). Thermal spray coating techniques have shown good results for high oxidation resistance which also include the Cr3C2-NiCr and WC-Co coatings sprayed with detonation gun spray method and this coating showed good adherence to the base metal in high temperature oxidation zone with no sign of spalling, and oxide scale are also formed during this aggressive environment (Kaur et al., 2011a; Kaur et al., 2011b).
This method is also termed as thin film deposition technique. The schematic diagram for performing this experiment is shown in Figure 6. The material to be deposited on the base material was ejected from the target in the form of vapour. The atoms sputtered from the target have a high energy distribution and inert gas of closer atomic weight to the target is used for efficient momentum transfer.
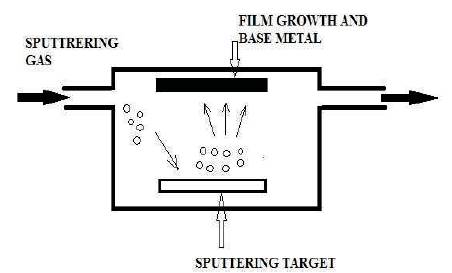
Figure 6. Schematic Representation of Sputter Deposition Process
The types of sputter deposition techniques are Ion-beam sputtering and Ion-assisted deposition. This process has certain disadvantages that it is difficult to control the atom's movement, which further lead to contamination problems. Tungsten carbide as a base metal for die has been coated with boron nitride and the structure was observed with infrared spectroscopy technique which reflected change in open air conditions; while using nitrogen environment in coating chamber helped the structure to remain same. Other studies related are the physical vapour deposition technique and this was used for coating of dies and shown good results with the coatings of platinum and irradium. Both these metals are noble metals and at the same time they are very costly as well. Irrespective of these noble metals, TiAlN coating was also tried by researchers with PVD technique, but it did not show any improvement in sticking behaviour and lowers the sticking temperature (Ghrib et al., 2009; Mayrhofer et al., 2001, 2002). Sputtering deposition technique showed good results with Pt-Ir coating and also had stability of coating with the use of nitrogen in coating chamber. However, in actual practice it is not feasible to introduce nitrogen in dies. Corrosion protection with physical vapour deposition has been studied by using TiAIN coatings in the form of nanograin coatings and it showed better results than conventional micron sized powder (Liscano et al., 2006; Lembke et al., 2000) and in another study, PVD coatings on AISI H13 were followed heat treatments and it also showed better wear resistance at high temperature. TiAlN and AlTiN are another stable nitrides that easily formed with Tungsten and aluminium and these nitrides sustain up to 800 °C and these have a good thermal conductivity (Liscano et al., 2006). The use of PVD TiAlN coatings help to withstand high temperatures due to alumina layer formation which is stable and passive even at 800 °C for high temperature corrosion (Choi and Park, 2006).
For claddings through microwave heating, there is an interaction of electromagnetic waves to heat the material and the heat is generated inside the material. The powder is placed on the top surface of the material. Due to heating, the powder merges into the surface of the substrate. The disadvantage of the process is that the surface produced after the process does not have good surface texture and needs surface finishing process for the surface to be used (Prasad and Gupta, 2013). The main advantage of this process is that it produces micro structural bonding between base metal and powder particles which gives very dense structure and having very less porosity. These good characteristics provide good wear and oxidation resistance due to closely packed structures. Figure 7 shows the representation of Microwave cladding process.
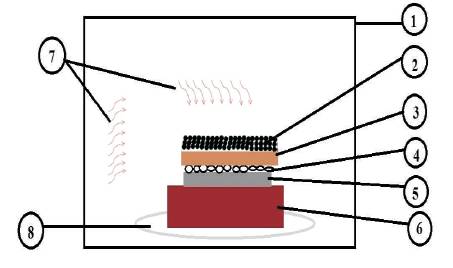
Figure 7. Schematic Representation of Microwave Cladding showing (1) Microwave, (2) Susceptor, (3) Separator, (4) Powder Grains, (5) Base Metal, (6) Brick, (7) Microwaves, and (8) Rotating Table
This is an emerging process in surface engineering and provides good resistant to erosion and wear with use of micrometric and nanometric WC–12Co powders and these powders provide efficient results, especially nanopowder gives much better results (Zafar and Sharma, 2016).
Coatings showed high hardness with low coefficient of friction and increased elasticity, which further needs to be improved (Sarhadi et al., 2014) and the elastic behaviour of the mould materials affect the final shape of the product to be moulded. Therefore, elasticity has been considered even in finite element analysis in order to precompensate for the moulds in advance (Tsai et al., 2008). Nickel chrome coatings were used on stainless steel for the manufacturing of precise glass items. The surface finish was maintained better than 50 nm average roughness (Firestone and Yi., 2005). Therefore the presence of nickel helps to retain the properties and maintain the surface texture and the quality of surface in glasses mainly depends on the surface of the mould and it is directly affected by the surface finish of the mould material (Wadsworth et al., 1997).
With addition of tungsten in CrN results in high hardness and Cr-N itself gives high toughness. Coatings of CrN, CrBN, and CrSiN deposited by sputtering process are unstable at high temperatures, which lead to the interaction between coatings and glass. However, nitrogen prevents the oxidation of the mould material and improves the sticking problems (Ma et al., 2008). While there is increase in mould temperature up to the critical temperature of mould material, sticking will occur and intensity of sticking increases as the temperature of mould increases. Thermal cycling behaviour of the glass mould surface was evaluated on uncoated stainless steel grades and coated surface boronized substrates (DIN 1.2080, DIN 1.2080 surface boronized, DIN 1.27870). It has been concluded that surface roughness changes with number of thermal cycles and thermal conductivity of the material (Hones et al., 2000). Pt-Ir coatings were also used on mould materials with PVD and magnetron sputtering techniques. The melting point of the Pt-Ir coatings is over 2000 °C and these are non-reactive with carbon. Due to high cost of noble metals like Pt-Ir, there is a continuous search for alternative coatings.
As mentioned in the above sections, various coating techniques have been used for coatings on working surface of dies. Efficient working surface of the glass moulds can be achieved by increasing the sticking temperature with relevant oxidation and wear resistant coatings which retain their properties even at higher temperatures. The glass manufacturing dies are designed for oxidation resistance, wear resistance, and high temperature strength which are achieved by proper selection of coating material and process. The following points have been concluded from this study:-
(i) Single layer coatings of nitrides, borides, oxides, such as TiBC, TiBCN, TiN, and NiAIN.
(ii) Noble metal (Pt-Ir) coatings.