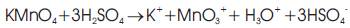
Graphene Oxide, the most recent nano scale form of carbonaceous material, has attracted much attention recently because of its unique electrical, thermal and mechanical properties, and its tremendous applications in different fields such as in optical, electronic, and catalytic fields. Graphite powder was used as a raw source for the synthesis of graphene oxide via modified Hummer's method. The structural and physiochemical properties of the synthesized product were investigated using techniques, Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Diffraction Spectroscopy (XRD) and Energy- dispersive X-ray spectroscopy (EDS). The FTIR analysis of graphene oxide was found to exhibit several characteristic absorption bands C=O, C=C, C-OH, and C-O suggesting that oxygen-containing groups were introduced into the graphene. The XRD pattern of the prepared graphene oxide showed a sharp peak centered at 2Ɵ =9.388, which resembled to the graphene oxide. The SEM micrographs of synthesized graphene oxide demonstrated the layered structure, which affords homogeneous ultra thin graphene films. The EDS analysis of the graphene oxide showed that the sample contains C and O, no other impurity was detected indicating the final product is free of impurities. C and O content was detected 60.90 and 39.10 weight% respectively.
Carbon nanostructures due to their excellent properties have immense applications in fields such as in optical, electronic, and catalytic fields [1, 2]. Nowadays, a new twodimensional (2D) monolayered carbon nanostructure parked into a dense hexagonal network structure, Graphene, has received tremendous attention from both experimental and theoretical scientific research communities [3]. Researchers have reported that by the hybridization of graphene with some materials, the recombination rate of electron–hole pairs decreases and the charge transfer rate increases, which makes graphene very useful in applications such as solar cells and photoelectron catalysts [4, 5]. Graphite is naturally occurring and available in large quantities and is inexpensive. Graphite mainly exists in three forms and these three types are different in their morphology and structure.
The types are namely, Lump Graphite (Vein graphite), Crystalline Flake Graphite (flake graphite), and the Amorphous Graphite [6]. Graphene does not exist naturally and therefore it is prepared using chemical methods. Graphene is a one atom thick planar sheet of sp2-bonded carbon atoms that are parked into a dense hexagonal honeycomb crystal lattice [7]. Graphene can be synthesized by four main methods. First method is the Chemical Vapor Deposition (CVD) and epitaxial growth, second method is micromechanical exfoliation of graphite, also known as the peel off method or the “Scotch Tape method”, third method is epitaxial growth on electrically insulating surfaces, fourth method is based on creation of colloidal suspensions [8, 9]. However, for large scale manufacture, these methods are less effective and it is difficult to achieve high-quality industrial production of graphene by these methods [10]. Therefore, in order to obtain high quality and bulk production of graphene, the simple, easy to handle, and cost effective route to synthesis of graphene is necessary. The chemical oxidation of graphite is the initial route employed to synthesis graphene. The most efficient method for the chemical oxidation of graphite, commonly known as Hummers' method involves the use of strong oxidizing agent such as potassium permanganate and potassium perchlorate for the oxidation of graphite powder to form Graphene Oxide (GO). The graphene oxide synthesized by Hummers’ method can be reduced using various reducing agents [11].
Benjamin C. Brodie, in 1859 first fabricated graphene oxide, he treated graphite powder with a mixture of potassium chlorate and fuming nitric acid. In 1957, Hummers and Offeman used a mixture of concentric sulfuric acid, sodium nitrate, and potassium permanganate to develop a quicker and safer process for the synthesis of graphene oxide. The Hummers' method involves oxidation of potassium permanganate and sulfuric acid (Equation 1). In this method, the active species diamanganese heptoxide (Mn2O7 ) is obtained via the reaction of monometallic tetraoxide (MnO4-) and MnO3+ (Equation 2). This diamanganese heptoxide (Mn2O7) (i.e. dark oil) which is more active species than permanganate, is placed in contact with organic compounds or heated up to temperatures greater than 55 °C [11,12].
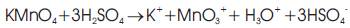
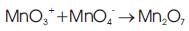
In the present work, an attempt has been made to synthesize graphene oxide with homogeneous ultra thin layers by the modified hummer's methods. The structural and physicochemical properties of the prepared graphene oxide have been described in this paper.
Graphite powder (100 micron) was purchased from S D fine-Chem Limited, Sulphuric acid (H2SO4 ) 98% Conc., Sodium Nitrate (NaNO3) and Sodium Hydroxide (NaOH) were purchased from Fisher Scientific, Hydrogen Peroxide (H2O2) (30%) was purchased from LOBA Chemie, and Potassium Permagnate (KMnO4) was purchased from Rankem. All the chemicals were of analytical reagent grade and were used as received without any further purification.
Graphene Oxide (GO) was synthesized from graphite powder using modified Hummer's method. In brief, 3 gm of graphite powder and 1.5 gm of sodium nitrate were mixed together followed by the addition of 69 ml of conc. sulphuric acid under constant stirring speed of about 400- 500 rpm. After 1 hour of constant stirring, 9 gm of KMnO4 was added slowly to the above solution while keeping the reaction temperature less than 20 °C to prevent overheating and explosion. To provide temperature less than 20 oC, reaction mixture was kept in cold ice water bath, as the addition of Oxidizing agent KMnO4 is an exothermic reaction and generate heat while adding. While addition of KMnO4 to the mixture of Graphite powder and Conc. sulphuric acid, the stirring speed was decreased because of the small explosion disturbances in the reactor. The mixture was then stirred at 35 °C for 12 hours and the resulting solution was then diluted by adding 1500 ml of cold distilled water under vigorous stirring for 1 hour. To ensure the completion of reaction of Graphite powder with KMnO4 , 30% H2O2 solution (15 ml) was added to the reaction mixture resulting in yellow-brown color indicating the completion of reaction and neutralization of unreacted and excess H2SO4 . The resulting mixture was then first filtered using filter cloth and then centrifuged at 4000 rpm for 1 hour with subsequent washing with 20% dilute HCl and distilled water respectively, followed by drying at 80o C for 1 hour [1,10,11].
Fourier Transform Infrared (FTIR) spectra of the samples were recorded on a Shimadzu FTIR-8400 spectrophotometer using KBr as the mulling agent. X-Ray Diffraction analysis (XRD) of graphene oxide was conducted on Advance X-ray diffractometer, D8, (Brukers Germany) with CuKα, radiation (λ = 1.5404 Å). Data were collected within the 2θ range of 20 – 80o at a scan rate of 0.1° min- 1 . The morphology was observed by a Field Emission Scanning Electron Microscope (FESEM, model S4800 type 2 HITACHI Tokyo Japan) at 10 keV. The samples were coated with gold before analysis. The Energy-Dispersive X-ray Spectroscopy (EDS) analysis of the Graphene Oxide was also taken on this FESEM.
Graphene oxide was successfully synthesized by treating graphite powder with concentrated acid in presence of strong oxidizing agent. Oxidation of graphite powder using strong oxidizing agent results in a yellow-brown colored slurry [11]. The slurry includes exfoliated sheets of graphene oxide along with residue of the oxidizing agents and nonoxidized graphite particles. The residue and non-oxidized graphitic particles are removed by repeated centrifugation and repeated washing with dilute HCl and water [13]. Graphene oxide can be electrostatically stabilized into suspension using water, alcohols and certain organic solvents and can be exfoliated into individual sheets by ultrasonic agitation overcoming the strong van der Waals interactions among the layers of graphene [14].
From FTIR spectrum, Graphene oxide was found to exhibit several characteristic absorption bands of oxygen- containing groups such as C=O stretching (1733.10 cm-1 ), C=C (1634.73 cm- 1), C-OH (1221.95 cm-1), and C-O stretching (1066.67 cm-1) functional groups, suggesting that oxygen-containing groups are introduced into the Graphene [15]. The absorption peaks are at 833.28 cm−1 for aromatic C-H deformation, 1,221.95 cm- 1 for phenolic C-OH stretching, and a broad peak at 3,621.47 cm-1 for the O-H stretching vibrations of C-OH groups. The small peaks at 2,753.37 and 3061.13 cm-1 in the spectrum were attributed to the CH2 stretching vibration [16]. Figure 1 shows the FTIR spectrum of graphene oxide.
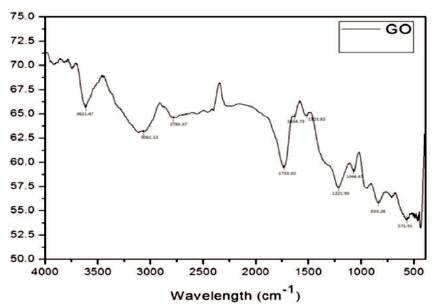
Figure 1. FTIR Spectrum of Graphene Oxide
Figure 2 shows the XRD pattern of the synthesized graphene oxide. The XRD pattern of the prepared graphene oxide showed a sharp peak centered at 2Ɵ =9.388, which resembled to the graphene oxide [17] and shows 61.1% crystallinity.
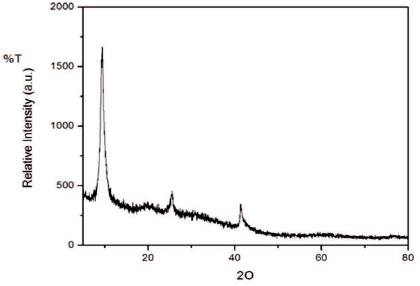
Figure 2. XRD Pattern of Graphene Oxide
Figure 3 shows the SEM micrographs of graphene oxide with different resolutions. From the Figure, it can be observed that graphene oxide has a layered structure, which affords ultra thin and homogeneous graphene films [1]. The films were folded or continuous at times including kinked and wrinkled areas.
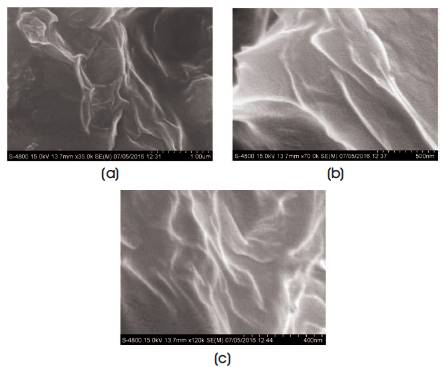
Figure 3. SEM Micrograph of Graphene Oxide (a) 1μm, (b) 500 nm, (c) 400 nm
Figure 4 shows the Energy-Dispersive X-ray Spectroscopy (EDS) analysis of the Graphene Oxide and it shows that the sample contains C and O, no other impurity was detected indicating the final product is free of impurities. C and O content was detected 60.90 and 39.10 wt% respectively. The carbon to oxygen atomic ratio was 1.55. This result indicated that the oxygen content was more than the empirical formula proposed by Boehm C6H2O3 [18] .
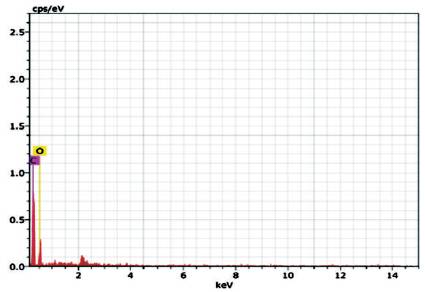
Figure 4. EDS Spectrum of Graphene Oxide
The graphene oxide was successfully synthesized from graphite powder by modified Hummer's method. The FTIR analysis of graphene oxide was found to exhibit several characteristic absorption bands C=O, C=C, C-OH, and CO suggesting that oxygen-containing groups were introduced into the graphene. The XRD pattern of the prepared graphene oxide showed a sharp peak centered at 2Ɵ =9.388 and interlayer spacing 9.41 Å which resembled to the graphene oxide. The SEM micrographs of synthesized graphene oxide demonstrated the layered structure, which affords homogeneous ultrathin graphene films. The EDS analysis of the graphene oxide showed that the sample contains C and O, no other impurity was detected indicating the final product is free of impurities. C and O content was detected 60.90 and 39.10 weight% respectively. The carbon to oxygen atomic ratio was 1.55.