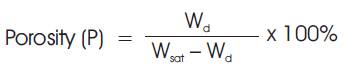
Ceramic whitewares practically signify vitreous or glassy elements which are usually formed from a mixture of China Clay, ball clay, quartz and feldspar milled to their possible finest particles and fired to form a ceramic compound. Ceramic whitewares are generally associated with some tremendous technical properties which make them applicable in nearly all fields of life. These admirable properties are usually a function of the China Clay present in the composition which varies respectively with their geological deposits, and furthermore affects the mechanical and physical properties of the composed whiteware. This research is therefore aimed at studying the variation of China Clay deposits and their effect in technical properties of ceramic whiteware compositions, for the production of ceramic whitewares. This was carried out by substituting China Clay of different deposits in a standard whiteware composition which was later fired at different temperatures of 1050oC, 1150oC, 1200oC and 1250oC respectively with a soaking time of 1 hour.
Abundance of the elements and their geochemical characteristics mainly control the types of minerals found in nature. Since oxygen, silicon, and aluminium together account for almost 90 per cent of the elements in the earth's crust, it is not surprising that the dominant minerals are quartz, silicates, and alumino silicates. These, together with other mineral compounds of oxygen, constitute the great bulk of naturally occurring ceramic raw materials. Thus, the mineral raw materials used in the ceramic industry are mainly inorganic, non-metallic and crystalline solids formed by complex geological processes (Thomas, 2014). Crystalline structure, chemical composition of the essential constituents and the nature and amounts of accessory minerals present largely determine ceramic properties. Consequently, these ceramic properties are subject to wide variation depending on the geological environment in which the mineral deposit was formed.
Clay is an abundant raw material found almost everywhere on earth. However, certain grade types of clay deposits are limited in geographic occurrence and extent. Clays containing a preponderance of the clay mineral kaolinite are known as kaolinitic clays. Meanwhile, certain commercial clays used in some part of the world are composed predominantly of kaolinite. These are China clays, kaolines, ball clays, fireclays and flint clays (Birks 2003). The terms China clay and kaolin are used interchangeably in industries by major experts in the field.
According to (Hoffman, 1991), Ceramic products are obtained as a result of a specific procedure of processing of clay raw materials, feldspars and flint. During wet preparation and forming stages, it is important that ceramic slurries and pastes show a good rheology, plasticity, and binding capacity for non-clay raw materials; all these properties influence the shrinkage, and thus the mechanical resistance of the products. These features depend on the nature, ratio and size of clay particles as compared to the non-clay materials. Conventional ceramics are obtained from clay minerals, feldspars, and flint. Clay minerals are provided by kaolines and other clays, and represent more than 50 % of the ceramic mass. They ensure a good behaviour of ceramic slurries at wet preparation and forming (plasticity, rheology), and finally they influence the properties of the firing product. The main mineral phases within clays are kaolinite, quartz, and micas. Carbonates and small amounts of feldspars, chlorites, montmorillonite, interstratified minerals, and compounds of Ti and Fe are considered to be impurities, some showing a strong negative effect on the final product. In general, the technological process was hardly adapted to mechanization and automation of certain flow stages, due to the compositional heterogeneity of natural raw materials. Modern methods of investigation allow a rigorous control of raw materials nowadays and accordingly, the possibility of adaptation of certain ceramic mass compositions to the existing materials. Alternatively, specific ceramic compositions can be recommended for proper types of final product. (Wilson, 1987).
According to Wilson (1996), whiteware is any of a broad class of ceramic products that are white to off-white in appearance and frequently contain a significant vitreous, glassy component.
Including products as diverse as fine China dinnerware, lavatory sinks and toilets, dental implants, and spark-plug insulators, whitewares all depend for their utility upon a relatively small set of properties; imperviousness to fluids, low conductivity of electricity, chemical inertness, and an ability to be formed into complex shapes. In whitewares and sanitary wares, kaolin provides a white body, gives easy molding properties, and adds strength, dimensional stability and smooth surfaces to the finished product. Refractoriness, dimensional stability and chemical inertness makes the kaolins uniquely suitable for special refractories.
The fact is that quartz, clay and feldspar relatively found in variations in whiteware body compositions are not too sensitive to minor changes in composition and forming methods, and firing temperature is one of their greatest advantages. The main differences between compositions are in the relative amounts and kinds of feldspar and kaolin used which are subject to deposits in different locations around the world. As an increasing amount of feldspar is added, the amount of liquid formed at the eutectic temperature increases, vitrification proceeds at a lower temperature due to this extra amount of liquid present and greater vitrification and higher translucency are obtained. As feldspar is replaced by clay, higher temperatures are required for vitrification and the firing process becomes more difficult and expensive, however the forming processes become easier, and the mechanical properties of the resulting body are improved (Olusola,1998). Requirements relating to forming the ware mostly determine the amount and kinds of clay selected. As more difficult forming techniques are employed, a larger clay content is required.
Whiteware products are often differentiated into three main classes as given below, according to their degree of verification and resulting porosity.
Proceeding from porous to vitreous, more particular product categories include earthenware, stoneware, China, and technical porcelains. Earthenware is nonvitreous and of medium porosity. It is often glazed to provide fluid impermeability and an attractive tile ware. Stoneware is a semivitreous or vitreous whiteware with a fine microstructure, that is, a fine arrangement of solid phases and glass at the mircometer level. The products include table ware, cookware and sanitary ware. All vitreous whiteware are often referred to as porcelains, but in the ceramics industry a distinction is maintained between the true porcelains or technical porcelains and China. China is known for high strength and impact resistance and also for low water absorption, all deriving from the high glass content. Typical products include impact resistance suiting it to commercial use; fine China tableware; and sanitary plumbing fixtures. Technical porcelains, like China, are vitreous and nonporous. They are similarly strong and impact resistant, but they are also chemically inert in corrosive environments and are excellent insulators against electricity. Applications include chemical ware, dental implants and electric insulators, including spark-plug insulators in automobile engines. (Ryan, 1987).
Porcelain wares such as those similar to the Yongle-era porcelain flasks, were often presented as trade goods during the 15th century Chinese maritime expeditions. Porcelain is a ceramic material made by heating materials, generally including clay in the form of kaolin, in a kiln to temperatures between 1,200 and 1,400 °C (2,200 and 2,600 °F). The toughness, strength, and translucence of porcelain arises mainly from the formation of glass and the mineral mullite within the fired body at these high temperatures (Shashidhar and Reed 1990).
Porcelain derives its present name from the old Italian word “porcellana” (cowrie shell) because of its resemblance to the translucent surface of the shell. Porcelain can informally be referred to as "China" or "fine China" in some Englishspeaking countries, as China was the birthplace of porcelain making. Properties associated with porcelain include low permeability and elasticity; considerable strength, hardness, toughness, whiteness, translucency and resonance; and a high resistance to chemical attack and thermal shock. For the purposes of trade, the Combined Nomenclature of the European Communities defines porcelain as being "completely vitrified, hard, impermeable (even before glazing), white or artificially coloured, translucent (except when of considerable thickness), and resonant." However, the term porcelain lacks a universal definition and has been applied in a very unsystematic fashion to substances of diverse kinds which have only certain surface qualities in common (Eurcaem, 2004).
Bone China is a type of soft paste porcelain that is composed of bone ash, feldsparthic material, and kaolin. According to Schneider (1991), bone ash can be defined as ware with a translucent body containing a minimum of 30% of phosphate derived from animal bone and calculated calcium phosphate. In his explanation, he said bone China is known for its high levels of whiteness and translucency, and very high mechanical strength and chip resistance. Its high strength allows it to be produced in thinner cross-sections than other types of porcelain. The production of bone China is similar to porcelain, except that more care is needed because of its lower plasticity and a narrower vitrification range. The traditional formulation for bone China is about 25% kaolin, 25% Cornish stone and 50% bone ash (Harry & Richerson 2002). The bone ash that is used in bone China is made from cattle bones that have a lower iron content. These bones are crushed before being degelatinised and then calcined at up to 1250 °C to produce bone ash. The ash is milled to a fine particle size. The kaolin component of the body is needed to give the unfired body plasticity which allows articles to be shaped. This mixture is then fired at around 1200°C. The raw materials for bone China are comparatively expensive, and the production is labourintensive, which is why bone China maintains a luxury status and high pricing. Bone China consists of two crystalline phases, anorthite (CaAl2Si2O8) and β-tricalcium phosphate/whitlockite (Ca3(PO4) 2) embedded in a substantial amount of glass. (Allen et al 1986).
The properties of ceramic whitewares are regulated not only by the composition and structure of the phases present but also by their arrangement. Normal triaxial whiteware bodies consist essentially of the following microscopic phases as described by (Hoffman, 1990):
Of course, this structure represents a series of arrested reactions or failure to attain equilibrium. However, the attainment of equilibrium is not necessarily desirable.
Maturation of the body is accelerated and the firing range is increased when the particle size of the components is reduced. Smaller particles also reduce warpage and increase translucency and mechanical strength (McLaren and Giordano, 2005). The particle size effects of the flint are relatively more pronounced than those of the feldspar. The expansion and contraction of quartz grains in the matrix lead to stresses that may give rise to actual cracking. The stresses in individual grains can be reduced if the grain size is reduced. The properties of porcelains are improved if finely grained quartz is used rather than coarse material. The crystal size of the microcrystalline silicas is much smaller than the apparent grain size produced by grinding. The converse generally holds for the macro crystalline types of silica. Thus, at temperatures between 1000oC and 1150oC, an appreciable amount of cristobalites are formed from flint in a body, whereas little or none can be detected in a quartzose body below 1200oC (Carty, 2002). Both the alpha to beta and beta to alpha cristobalite changes occur over a relatively wide range of temperatures, and are therefore probably less potent causes of dunting (cooling cracks) than the quartz conversions. Finer grinding of the feldspar leads to greater glass development, reduced mullite occurrence in the feldspar pseudomorphes, fewer closed pores and improved translucency.
Clay is the most important of the ingredients used in making ceramic whiteware, and the most important clays used is kaolin, also known as China clay. Kaolin is the only type of clay from which a white, translucent, vitreous ceramic can be made. It is a refractory clay, meaning that it can be fired at high temperature without deforming. For the purpose of this study, China clays of different properties from different locations in southern western part of Nigeria were basically chosen in order to conduct a comparative study in detecting the best deposit for ceramic whiteware production. The other raw materials used like silica, feldspar, ball clay and calcium carbonate were also gotten in south western part of Nigeria. Kaolin, which is the principal raw material was gotten from three different deposits, which are: Afowa (Edo state), Okpillar (Edo-State) and Ikere (Ekiti State), while feldspar was gotten from Okpillar in Edo State.
A standard whiteware body composition for the formulation of the selected nine body batches, with major emphases on different China clay deposits is used. Other valuable whiteware forming materials like feldspar which reduces the vitrification time of the composition, quartz which supplies silica and also gives the composition strength, ball clay which provides plasticity and moreover binds these materials together, and finally calcium carbonate which serves as flux as well as increasing the brightness features of the whiteware body composition. All these materials were added accordingly following the standard body composition as shown in Table 1. These materials were carefully weighed using a digital scale. The weighed raw materials were charged into a ball mill containing porcelain grinding pebbles of different size and weight, 35% water of the total weighed body batch was also added for wet milling purpose with an addition of sodium silicate also known as water glass, which serves as deflocculant. The ball mill was powered to constantly roll for three hours before its content was discharged completely. The milled slurry was further dried in a muffled drier at 110 ºC for three hours. The dried samples were properly weighed and 7% of water was added to make it suitable for dry pressing. A hydraulic jack of 10 ton capacity was used for the dry pressing. This was actualized by measuring each sample into iron steel mould of 100mm by 100mm which was used for shaping the samples into the said dimension. The mould was lubricated and the samples were measured accordingly in order to get equal thickness and size before pouring inside the mould, it was then subjected to the hydraulic jack by applying pressure so as to form a compacted ceramic tile of equal length and width. After the dry pressing, the samples were allowed to dry at room temperature and later placed in the kiln for firing at four different temperatures 1050oC, 1100oC, 1200oC and 1250oC under reduction atmosphere and maintained for an hour for soaking purposes and further left to naturally cool before offloading them.
In order to characterize the fired samples, different tests were carried out using standard procedures. Some of the test used for characterizing the samples include: Porosity test, Water absorption test, Shrinkage test and Flexural strength test.
The boiling method was used for this test at 100ºC for 2 hours. The samples were subjected to 1 hour boiling followed by additional two hour water soaking and then weighed as Wsat. The soaked samples were then suspended from the beam of a balance in a vessel of water so arranged that, sample was completely immersed in the water without touching the side of the vessel. The suspended sample in water weighed Wsus. Porosity was then calculated by the equation (1).
The porosity affects a number of properties of ceramic materials but probably the most important effect is on its strength. The lowest porosity has the greatest strength. Porosity was determined by the boiling method which is the standard procedure. Figure 1 shows the values of porosities of samples from Samples A to I varying for different temperatures. The porosity of the fired sample is associated to other physical properties such as shrinkage, bulk density and water absorption. Table 1 shows the former properties as function of firing temperature at the range of 1050–1250˚C. Water absorption is directly related to open porosity, its value decreases in the overall temperature range and the variation of mechanical properties with porosity for the composition with different kaolin particle size. The occurrence of 45% kaolin does not substantially change the values of water absorption and open porosity, which decreases sensitively when 40% kaolin is added. Only samples of the series A to D (Table 1) shows, how an appreciably important change in their mechanical behavior occur as they reach 1250˚C.
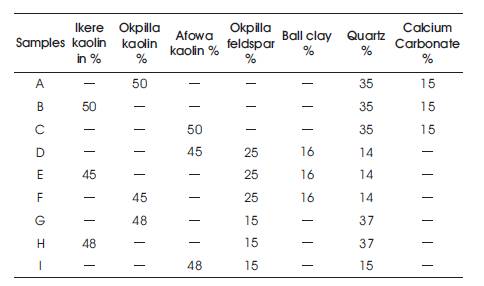
Table1. Mixture of Composition
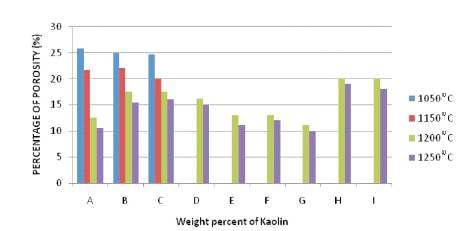
Figure 1. Percentage of Porosity of the Samples
The produced samples were tested for its quality assessment. Physical properties (porosity and water absorption) of the specimen were conducted by boiling water method.
Water absorption is related to the microstructure of a sintered ceramic matrix, and evaluates the open pores amount of the fired specimen. Water absorption was then calculated by equation (2),
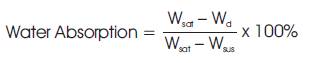
Where, Wsat= Saturated weight, Wd=Dry weight. Wsus = Suspended immersed weight.
The internal structure of the ceramic whiteware body must be compact enough to avoid the intrusion of water. Water absorption is used to estimate the pore ratio of ceramic whiteware samples. High water absorption in whiteware is characterized by a high pore ratio, which requires the water absorption of ceramic bodies to be less than 16% (From Figure 2 it is known that all the samples with addition of kaolin resulted in a highest firing temperature only for the specimen SA that reaches values of approximately 15% (SA) at 1200˚C). The samples of ceramic whiteware bodies from different proportions of kaolin produced different values of water adsorption are thus compared
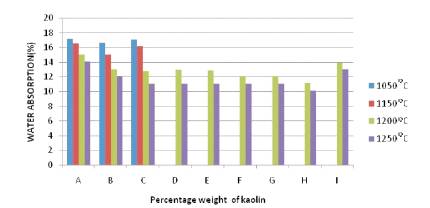
Figure 2. Percentage of Water Absorption of the Samples
The shrinkage test was carried out on the samples by determining the size before and after firing, using digital vernier caliper.
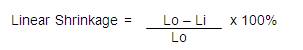
where Lo is the initial length of ceramic tiles, while Li is the fired length of the ceramic tiles.
The sample B which has the highest clay content with respect to other bodies showed the highest shrinkage value. And the compositions, with quartz added, as expected, showed small shrinkage. The firing behavior shown by the compositions A to G present an increase of shrinkage from 1150 to 1200˚C, that indicate an over firing, which occurs from 1150 to 1250˚C in D, E, F (Figure 3). It means that the highest amount of feldspar extends the firing range.
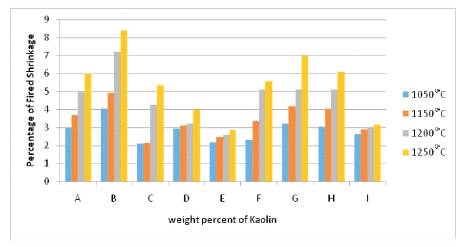
Figure 3. Percentage of Fired Shrinkage of the Samples
The flexural strength was determined using a universal testing machine (MTS 810.23M), in three-point bending fixture, 70 mm support span and with a crosshead speed of 0.5mm min-1 .
The flexural strength of the samples is shown in Figure 4. Flexural strength of the samples was examined in terms of Modulus Of Rupture (MOR).The mechanical behavior is quite similar to the vitrification pattern of the samples, i.e. MOR values increase with densification, with the sample containing highest percentage of kaolin fired at 1250oC exhibiting the highest flexural strength, while the samples fired at 1050oC exhibiting the lowest flexural strength. The MOR values of the sample F and G at temperature of 1250oC are between 36.3MPa and 38.4 MPa, respectively has been acceptable according to ISO standard that requires values not less than 35MPa.
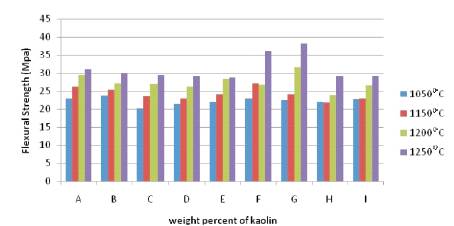
Figure 4. Flexural Strength (Mpa) of the Samples
Having composed several whiteware bodies by means of standard compositions using China clays from selected deposits in Edo and Ekiti states and conducted several mechanical property tests on the composed bodies in various temperatures 1050oC, 1150oC, 1200oC, and 1250oC respectively, it can be deduced that China clay from Okpella in Edo State (Samples A and G) produced the most desired result of brilliant white at 1200oC and 1250oC, with calcium carbonate and feldspar used simultaneously in both temperatures, and also produced a better result in mechanical property tests. Ikere Kaolin on the other side produced some astonishing result at much lower temperature at 1150oC indicating the availability of fluxes in the China Clay. Whiteware bodies can therefore be produced with samples C, I, E, H at low temperature showing that Ikere kaolin, Okpillar kaolin and Afowa kaolin can be used to produce white ware bodies at different composition with different desirable mechanical properties. Therefore table wares, sanitary wares and ceramic tiles can successfully be produced with material sources in South Western part of Nigeria.