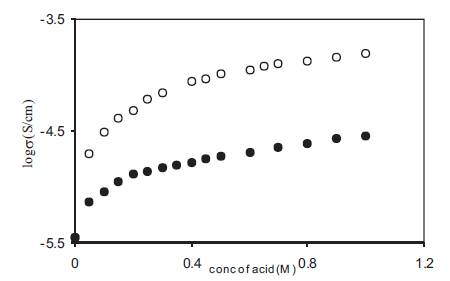
Figure 1. Variation of log conductivity with acid concentration for liquid electrolytes containing benzoic acid (•), and o-hydroxy benzoic acid (o) in DMA.
Composite proton conducting non aqueous polymer gel electrolytes have been synthesized by dispersing nano sized fumed silica to the polymer gel electrolytes containing polymethylmethacrylate (PMMA), dimethylacetamide (DMA), benzoic acid (BA) and ortho-hydroxy benzoic acid (o-OHBA). These electrolytes have been characterized by complex impedance spectroscopy, viscosity and pH measurements. The effect of acid, polymer and fumed silica on conductivity, pH and viscosity has been studied for gel electrolytes. Maximum conductivity of 2.95 x 10-4 S/cm and viscosity of 1.64 x 105 mPas at 25o C has been obtained. The conductivity of composite gels does not show any appreciable change with time and only a small change in conductivity is observed over the operational range of temperature, which is desirable for their use in device applications.
Fuel cells are projected as the clean energy sources of next generation and depending upon the electrolyte used, different types of fuel cells are available in the market [1]. Proton exchange membrane fuel cells (PEMFCs) which use a solid polymer membrane as the electrolyte are favoured for stationary, portable and mobile applications as these can operate at normal temperatures [2]-[5]. The most important part of PEMFCs is the membrane, which must conduct protons and should have good mechanical properties. The Nafion membrane presently used is very costly and is suitable for limited temperature range applications due to its humidity dependent conductivity. Hence, there is a growing need to develop an alternate proton conducting membrane which have high value of ionic conductivity and can be used over wide temperature range. Polymers containing functional acidic groups in the hydrated state [6] and in hetrocycles such as imidazole, pyrazole or benzimidazole [7] have also been reported. Proton conducting polymer gel electrolytes containing weak acids–namely aromatic carboxylic acids and aliphatic dicarboxylic acids have recently been reported o to show high value of conductivity at 25 C and being nonaqueous in nature, could be used over wide range of temperature [8]-[10]. Another advantage of these materials is that the addition of different polymers has been reported to result in an increase in conductivity along with an increase in viscosity. The dimensional stability of these polymer gel electrolytes can be further improved by dispersing small size insulating particles in these electrolytes. The aim of the present work is to develop nano dispersed non-aqueous polymer gel electrolytes with high value of conductivity and good dimensional stability which could find applications in PEMFCs and other devices.
In the present study, nano dispersed polymer gel electrolytes which are obtained by dispersing nano sized fumed silica, to proton conducting non-aqueous polymer gel electrolytes containing polymethylmethacrylate (PMMA) in the solutions of ortho-hydroxy benzoic acid in dimethylacetamide (DMA) has been investigated. The effect of the concentration of acid, polymer and fumed silica as well as temperature on the conductivity and viscosity of electrolytes has been studied. The effect of the molecular weight of the polymer on the conductivity and viscosity behaviour of polymer gel electrolytes has also + been studied. The change in free H concentration in the electrolytes has also been monitored by pH measurements.
Polymethylmethacrylate (PMMA) (Aldrich) with average molecular weights 15,000; 120,000 and 996,000; dimethylacetamide (DMA) (Merck) (ε = 37.8, ƞ = 1.937 cP, M.P. = -20o C, B.P. = 165o C), benzoic acid (C6H5COOH) 6(Lancaster) ortho-hydroxy benzoic acid (C6H4OH COOH) (Lancaster) and fumed silica (Aldrich) with surface area 380 m2 /g and grain size 7 nm were used as the starting materials. Liquid electrolytes were prepared by dissolving benzoic acid and ortho-hydroxy benzoic acid in different concentrations (expressed in molarity values) in DMA and polymer gel electrolytes were obtained by adding PMMA in different amounts (expressed as wt% of liquid electrolyte) to the liquid electrolytes along with continuous stirring by magnetic stirrer. Nano-dispersed gels were then prepared by dispersing nano sized fumed silica in different concentrations (expressed as wt% of polymer gel electrolytes) to the polymer gel electrolytes along with continuous stirring to ensure homogenization. Electrolytes containing different concentrations of ortho-hydroxy benzoic acid, PMMA and fumed silica have been studied in the present work.
The electrical conductivity of electrolytes was measured by complex impedance spectroscopy using HP4284A precision LCR meter in the 20Hz-1MHz frequency range with a cell having platinum electrodes. The conductivity was o also measured in the 20–90o C temperature range by using a temperature controlled furnace in steps of 5o C. pH of the electrolytes was measured by systronics 335 pH meter. The viscosity of different electrolytes was measured by Fungilab rotating viscometer (Visco Basic L) at different temperatures in water circulator (Julabo F12-EC) which could control the o temperature with an accuracy of ± 0.1o C.
The conductivity of liquid electrolytes obtained by adding benzoic acid and ortho–hydroxy benzoic acid (o-OHBA) to o dimethylacetamide (DMA) was measured at 25o C as a function of acid concentration and the results are given in Figure 1. The conductivity of solvent (~10-6 S/cm) increases by two orders of magnitude with the addition of acid and reaches a value of 1.55 x 10-4 S/cm for liquid electrolytes containing 1M o-OHBA. The acid upon dissociation provides free H+ ions which take part in the conduction process and as a result conductivity increases. However, the rate of increase of conductivity with acid concentration in not constant and is less at higher acid concentrations which is generally explained to be due to the formation of ion aggregates which do not take part in the conduction process. Although the solvent used (DMA) in the present o study has high dielectric constant (ε = 37.8 at 25o C) yet some undissociated acid will also be present in these liquid electrolytes as the acid used is a weak acid with dissociation constant (1.40 x 10-3) less than one in an aqueous solution. Due to lower dielectric constant of DMA as compared with water (ε=80) the amount of undissociated acid will be higher in liquid electrolytes containing DMA.

Figure 1. Variation of log conductivity with acid concentration for liquid electrolytes containing benzoic acid (•), and o-hydroxy benzoic acid (o) in DMA.
For comparison the conductivity variation for electrolytes containing benzoic acid is also given in Figure 1. The lower conductivity of electrolytes containing benzoic acid is due to its low dissociation constant (8 x 10-5 ) which is less than that for o-hydroxy benzoic acid and as a result benzoic acid is not fully dissociated in the solvent.
The increase in free H+ ion concentration with the addition of o-OHBA in liquid electrolytes has been studied by pH measurements and the variation of pH with acid concentration is given in Figure 2. pH decreases with an increase in acid concentration. As pH in an aqueous solution is given by pH = -log [H+ (aq.)] so a decrease in pH corresponds to an increase in acidity or an increase in free H+ ion concentration. A comparison of Figure 1 and Figure 2 shows that a decrease in pH is accompanied by a corresponding increase in conductivity. At high acid concentrations, the rate of decrease of pH with acid concentration also decreases. Although the electrolytes used in the present case are non-aqueous in nature yet a decrease in pH shall correspond to an increase in free H+ ion concentration in the electrolyte. The addition of acid to DMA also results in an increase in viscosity of the electrolyte and the variation of viscosity with acid concentration is given in Figure 3. Viscosity increases linearly with the concentration of acid but due to only a small increase in viscosity, its effect on conductivity shall also be negligible.
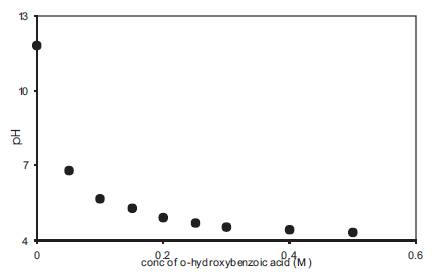
Figure 2. Dependence of pH on acid concentration for liquid electrolytes containing o-hydroxy benzoic acid in DMA.
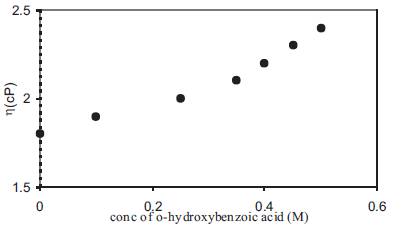
Figure 3. Change of viscosity with acid concentration for liquid electrolytes containing o-hydroxy benzoic acid in DMA.
Although liquid electrolytes possess high value of conductivity (~10-4 S/cm) at 25o C, yet they cannot be used in various devices due to their liquid nature. The polymer gel electrolytes obtained by immobilizing liquid electrolytes with the addition of a suitable polymer matrix can partly overcome the above drawbacks of liquid electrolytes. Polymer gel electrolytes were prepared by adding PMMA with average molecular weight 15,000 to liquid electrolytes containing 0.5 and 1M o-OHBA in DMA and the variation of conductivity at 25o C as a function of PMMA concentration is given in Figure 4. The conductivity of liquid electrolytes containing 0.5 as well as 1M o-OHBA increases with the addition of PMMA, reaches a maximum value at around 3–4wt% PMMA and then shows a small decrease at higher PMMA concentrations. The addition of polymer generally results in an increase in viscosity11-13 ) which shall lower ionic mobility and secondly, the addition of polymer also results in a net decrease in acid concentration as no additional acid has been added along with PMMA and both these factors – lower mobility and lower acid concentration shall result in lower conductivity. Despite this, the conductivity of liquid electrolytes increases with the addition of PMMA and this increase although small but, is quite significant and σ(gel) > σ(liquid) has been observed at all PMMA concentrations [12]-[13].
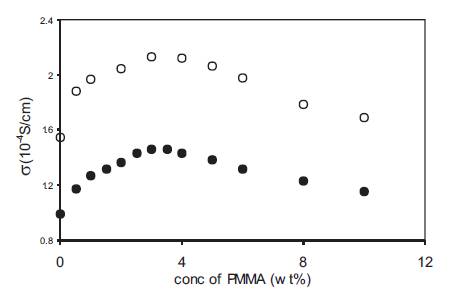
Figure 4. Variation of conductivity with PMMA concentration for polymer gel electrolytes containing 0.5M (•), 1M (o) o-hydroxy benzoic acid in DMA.
The presence of a maxima in the conductivity vs PMMA concentration plot (Figure 4) indicates the simultaneous presence of two competing processes in these gel electrolytes which are free ion concentration at low PMMA concentration and viscosity at high PMMA concentrations [14]. This was experimentally checked by pH and viscosity measurements on these polymer gel electrolytes. The variation of pH of gel electrolytes containing 0.5M o-OHBA as a function of PMMA concentration is given in Figure 5. pH decreases with an increase in PMMA concentration and the decrease is more upto about 3wt% and thereafter the decrease in pH is very small. The low PMMA concentration range in which pH decreases corresponds to the region in which conductivity increases with PMMA addition as given in Figure 4. The decrease in pH corresponds to an increase in free H+ ion concentration which contributes to an increase in conductivity. As the acid used in the present study is a weak acid, some undissociated acid is present in + these gel electrolytes and the increase in free H ion concentration is possibly due to the dissociation of undissociated acid with the addition of PMMA. The small change in pH at high PMMA concentrations is an expected behaviour because the amount of undissociated acid present in liquid electrolytes is fixed and with the addition of PMMA some of the undissociated acid gets dissociated resulting in a decrease in the amount of undissociated acid. This shall lead to a small change in pH at higher PMMA concentrations.
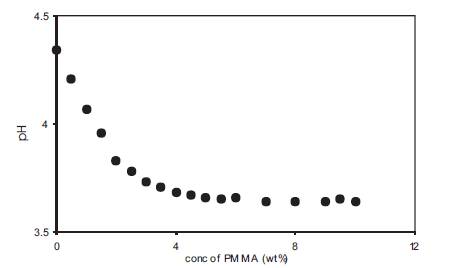
Figure 5. Dependence of pH on PMMA concentration for polymer gel electrolytes containing 0.5M o-hydroxy benzoic acid in DMA.
The viscosity of polymer gel electrolytes containing 0.5M o- OHBA was also measured as a function of PMMA concentration and the results are given in Figure 6. The viscosity increases linearly with PMMA concentration and the decrease in conductivity observed in Figure 4 at high PMMA concentration is due to the dominant role played by viscosity which is large at high PMMA concentrations. Although viscosity increases with increase in PMMA concentration yet its low value is due to the use of PMMA with low molecular weight (15,000). The role of viscosity in conductivity modification of polymer gel electrolytes was also investigated in more detail. However it may be pointed out that the viscosity measured experimentally by rotating viscometer is the macroscopic viscosity whereas it is the microscopic viscosity which is related to ionic mobility and hence conductivity. Literature reports on different polymer gel electrolytes report that the addition of polymer increases the viscosity of gel electrolytes which is accompanied by a small decrease in conductivity [11]. The conductivity is related to the viscosity by the relation
which suggests that an increase in viscosity shall lead to a decrease in conductivity.
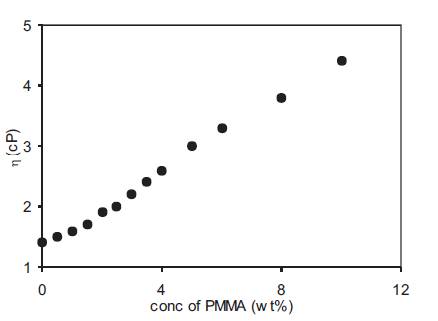
Figure 6. Change of viscosity with PMMA concentration for polymer gel electrolytes containing 0.5M o-hydroxy benzoic acid in DMA.
Further the viscosity of a particular polymer also depends upon its average molecular weight and it increases with an increase in the molecular weight of the polymer. Thus the viscosity of polymer gel electrolytes shall also depend upon the molecular weight of the polymer used. As pointed out in the above section, the viscosity of electrolytes plays a dominant role in conductivity modification at high polymer concentrations. So the use of polymer with different molecular weights shall affect the conductivity by different amounts. This was studied in the present case by using PMMA with average molecular weight 15,000; 120,000 and 996,000 [12]. Polymer gel electrolytes were prepared by adding PMMA with different molecular weights to the 0.5M solution of o-OHBA in DMA and the variation of conductivity with PMMA concentration is given in Figure 7. The conductivity of liquid electrolytes increases with the addition of PMMA having different molecular weights and the variation is similar to that observed in Figure 4. However the increase in conductivity observed with PMMA addition depends upon the molecular weight of PMMA used and is less for PMMA with higher molecular weights. However the conductivity of all the gel electrolytes containing PMMA with different molecular weights is higher than the corresponding liquid electrolyte at all concentrations of PMMA. Similar type of behaviour observed in the variation of conductivity with PMMA concentration for gel electrolytes containing PMMA with different molecular weights suggests that same type of mechanism is responsible for conductivity modification in these + electrolytes. At low PMMA concentration, free H ion concentration plays a dominant role whereas at high PMMA concentration, viscosity plays a dominant role in conductivity behaviour.
pH of polymer gel electrolytes containing PMMA with different molecular weights was also measured as a function of PMMA concentration and the results are given in Figure 8. In each case, the pH decreases at low PMMA concentrations and shows a near saturation value at high PMMA concentrations. However the extent of decrease in pH is also related to the molecular weight of PMMA used and the decrease in pH is more for gel electrolytes containing low molecular weight (15,000) of PMMA. A comparison of the results in Figure 7 and Figure 8 shows that an increase in conductivity with PMMA addition also leads to decrease in pH which is due to an increase in free H+ ion concentration which can take place due to the dissociation of undissociated acid present in these gel electrolytes containing weak acid. However as free ion concentration and viscosity are simultaneously affecting the conductivity behaviour so the use of high molecular weight PMMA (120,000 and 996,000) results in higher viscosity and as a result, the decrease in pH is small as compared with gel electrolytes containing PMMA with low molecular weight (15,000).
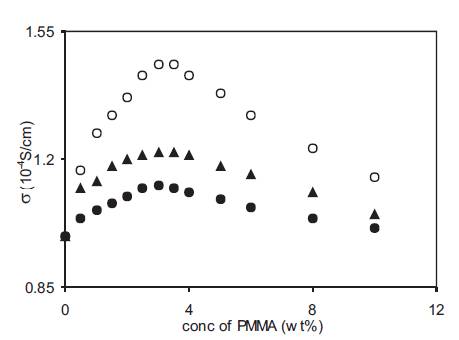
Figure 7. Dependence of conductivity on PMMA (av. mol. wt. 15,000 (o), 120,000 (▲) and 996,000 (•) concentration for polymer gel electrolytes containing 0.5M o-hydroxy benzoic acid in DMA.
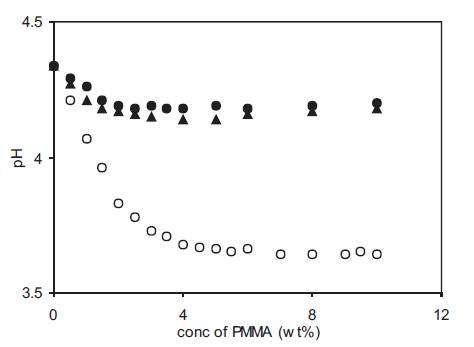
Figure 8. Variation of pH with PMMA (av. mol. wt. 15,000 (o), 120,000 (▲) and 996,000 (•) concentration for polymer gel electrolytes containing 0.5M o-hydroxy benzoic acid in DMA.
The dependence of viscosity of polymer gel electrolytes upon the molecular weight of PMMA used was also studied by measuring the viscosity of polymer gel electrolytes containing PMMA with different molecular weights and the results are given in Figure 9. The viscosity of gel electrolytes depends upon the molecular weight of PMMA used and is higher for gels with high molecular weight PMMA. In each case, at low PMMA concentrations the viscosity is low whereas at high PMMA concentrations the viscosity is very large and plays a dominant role and results in a decrease in conductivity as observed in Figure 7. Thus the results of the variation of conductivity, pH and viscosity as given in Figures 7–9 for polymer gel electrolytes containing PMMA with different molecular weights are compatible with each other. In each case the increase in free H+ ion concentration at low PMMA concentration is responsible for an increase in conductivity whereas the large viscosity at high PMMA concentrations plays a dominant role and results in a decrease in conductivity.
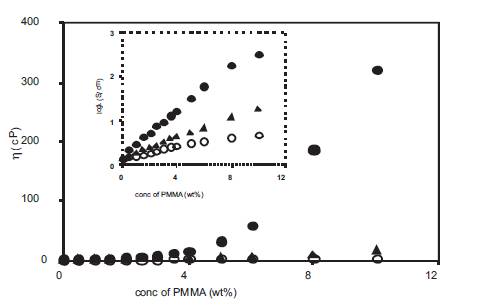
Figure 9. Variation of viscosity with PMMA (av. mol. wt. 15,000 (o), 120,000 (▲) and 996,000 (•) concentration for polymer gel electrolytes containing 0.5M o-hydroxy benzoic acid in DMA. (inset shows the variation of log viscosity with PMMA concentration).
The effect of the dissociation constant of the acid used on the conductivity behaviour of polymer gel electrolytes was also studied by synthesizing polymer gel electrolytes containing benzoic acid. Figure10 show the variation of conductivity with PMMA (av. mol. wt. 15,000) concentration for polymer gel electrolytes containing 1M benzoic acid in DMA. The conductivity increases with the addition of PMMA and gel electrolytes with conductivity higher than the corresponding liquid electrolytes are obtained for all concentrations of PMMA. However the increase in conductivity at low concentrations of PMMA is very large and is by 1-2 orders of magnitude as compared with that observed in Figure 4 for gel electrolytes containing ohydroxy benzoic acid. Secondly, the conductivity does not show a decrease at high PMMA concentrations as observed for o-OHBA although the rate of increase of conductivity decreases at high concentrations of PMMA. This could be explained as follows:
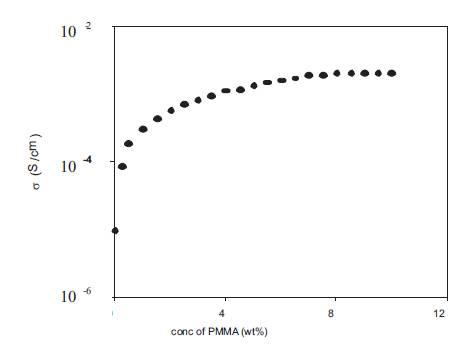
Figure 10. Variation of conductivity with PMMA (av. mol. wt. 15,000) concentration for polymer gel electrolytes containing 1M benzoic acid in DMA.
The benzoic acid used has very low value of dissociation constant (8 x 10-5 ) which is even less than that of o-OHBA and as a result a large amount of undissociated acid shall be present in these electrolytes. As the addition of PMMA results in the dissociation of undissociated acid so the increase in free ion concentration will be large and shall play a dominant role at all PMMA concentrations upto 10wt%. The effect of viscosity at high PMMA concentrations does not play a dominant role due to the dominant role of free ion concentration.
The change in free H+ ion concentration in polymer gel electrolytes containing benzoic acid was studied by pH measurements and Figure11 gives the variation of pH as a function of PMMA concentration for polymer gel electrolytes containing 1M benzoic acid in DMA. pH shows a linear decrease with an increase in PMMA concentration which shows that the concentration of H+ ion increases with PMMA addition upto 10wt% and this is reflected in the increase in conductivity with PMMA addition as given in Figure10. Thus in electrolytes containing benzoic acid, the amount of undissociated acid is large and the addition of PMMA results in the dissociation of benzoic acid even upto 10wt% PMMA.
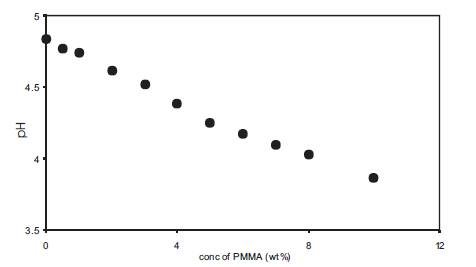
Figure 11. Change of pH with PMMA (av. mol. wt. 15,000) concentration for polymer gel electrolytes containing 1M benzoic acid in DMA.
On the basis of the above studies, it has been found that polymer gel electrolytes obtained by adding PMMA with molecular weight 15,000; 120,000 and 996,000 to the 0.5 and 1M solutions of o-OHBA in DMA show high conductivity as well as higher viscosity at 25o C than the corresponding liquid electrolytes. Despite this, a further improvement in the dimensional stability of these polymer gel electrolytes shall be needed for their use as electrolytes in various devices.
In 1973, Liang reported that the addition of an insulating material (Al2O3 ) to a poor ionic conductor (LiI) resulted in an increase in conductivity and these are known as composite electrolytes [15]. The increase in conductivity was later found to depend upon the particle size (the increase in conductivity was more for smaller particles) and concentration of insulating matrix [16]-[20]. Later on in the case of composite polymer electrolytes, the addition of insulating particles to the polymer electrolytes was also reported to result in an increase in the mechanical properties along with conductivity enhancement [21]-[26]. A similar approach has been employed in the present case to improve the mechanical strength of polymer gel electrolytes. Fumed silica with large surface area of 380m2 /g and having grain size 7 nm has been used in the present study as an insulating matrix.
Nano dispersed polymer gel electrolytes were obtained by adding fumed silica in different concentrations (expressed as wt% of polymer gel electrolytes) to the polymer gel electrolytes containing 10wt% PMMA with average molecular weight 15,000 in the 0.5 and 1M solutions of o-OHBA in DMA. The conductivity was measured at 25 C as a function of the concentration of fumed silica and the results are given in Figure 12 for electrolytes containing 0.5 and 1M o-OHBA respectively. The results show the presence of two maxima – one at very low concentrations (<1 wt%) of fumed silica and the second at ~8wt% fumed silica. The measurements have been made upto 10wt% fumed silica only as the composite gel electrolytes become highly viscous and more silica could not be added. Two maxima in the conductivity behaviour has also been reported earlier for composite polymer electrolytes PEO – NH4I – Al2O3[27], (PEO)8 – LiClO4 – SiO2 and (PEO)8 - LiN(CF3SO2)2 – SiO2[28], RbHSO4 – SiO2[29], (PEO)9 – LiCF3SO3– Al2O3[30] and (PEO)10 –LiClO4 – SiO2 [24].
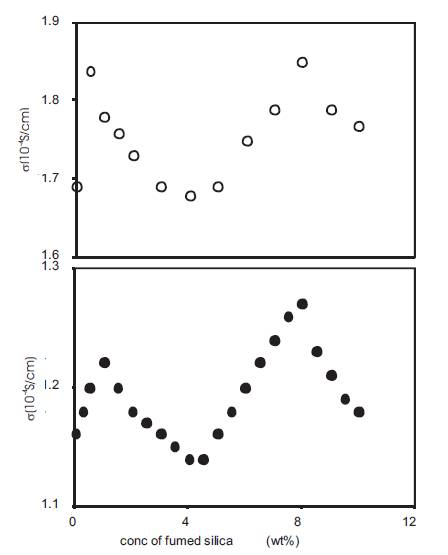
Figure 12. Conductivity variation with fumed silica concentration for nano-dispersed polymer gel electrolytes containing 10wt% PMMA in solution of 0.5M (•), 1M (o) o-hydroxy benzoic acid in DMA.
While studying the variation of conductivity of polymer gel electrolytes with PMMA concentration, the results of Figure 4 show that the conductivity is maximum for polymer gel electrolytes containing 4wt% PMMA. In order to obtain optimum value of conductivity, polymer gel electrolytes containing 4wt% PMMA were also used to prepare nano dispersed polymer gel electrolytes. The conductivity of nano dispersed polymer gel electrolytes containing 4wt%PMMA in 0.5 and 1M solutions of o-OHBA in DMA was also measured as a function of the concentration of fumed silica and the results are given in Figure13. The variation of conductivity is similar to that for gels containing 10wt% PMMA and two maxima at approximately the same concentrations of fumed silica are observed. Thus nano dispersed polymer gel electrolytes containing different concentrations (0.5 and 1M) of ortho-hydroxybenzoic acid and containing different (4 and 10wt%) amounts of PMMA with average molecular weight 15,000 show similar conductivity behaviour as a function of fumed silica concentration. The presence of two maxima in the variation of conductivity of nano dispersed gels with fumed silica could be explained as follows:
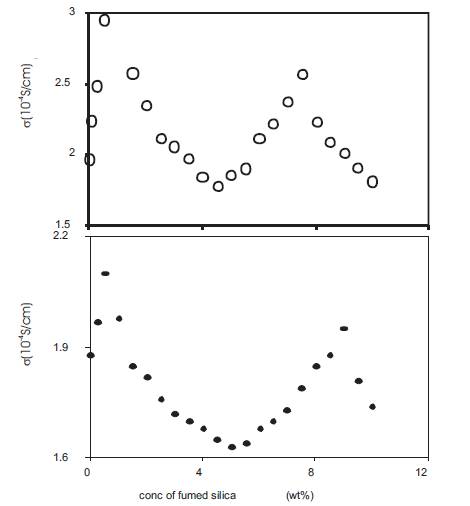
Figure 13. Conductivity variation with fumed silica concentration for nano-dispersed polymer gel electrolytes containing 4wt% PMMA in solution of 0.5M (•), 1M (o) o-hydroxy benzoic acid in DMA.
The presence of the first maxima at very low fumed silica concentration (<1wt%) is due to an increase in the dissociation constant of acid with the addition of nano sized fumed silica. Due to the charged surface of fumed silica particles, the H of hydroxyl (-OH) group in o-hydroxy benzoic acid shall be attracted towards the oxygen of fumed silica. This shall lead to an increase in the inductive effect which shall result in an increase in the dissociation constant of the acid [31]. This shall lead to an enhanced dissociation of the acid. The enhanced dissociation of the weak o-OHBA acid present in these electrolytes shall contribute to an increase in free H+ ion concentration and as a result an increase in conductivity is observed. However one could miss this maxima if measurements at very small concentrations of fumed silica are not taken. Irrespective of the concentration of o-OHBA and PMMA, this maxima is observed in each of the four systems studied in the present case.
The presence of the first maxima at very low concentrations of the fumed silica was also checked by studying polymer gel electrolytes containing benzoic acid which does not contain any hydroxyl group. Figure14 shows the variation of conductivity with the concentration of fumed silica for nano dispersed gel containing 10wt% PMMA in the 1M solution of benzoic acid in DMA. It does not show any maxima at low concentrations of fumed silica as observed for electrolytes containing o-OHBA. pH of the electrolytes also does not show any decrease with the addition of fumed silica in this case. Thus the addition of fumed silica does not enhance the dissociation of benzoic acid as observed in the case of electrolytes containing o-hydroxy benzoic acid.
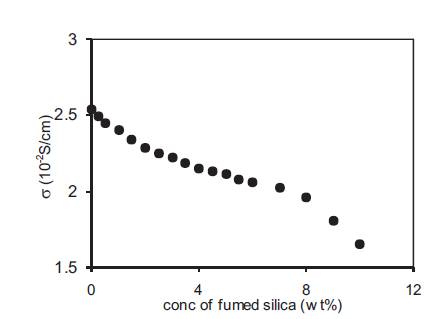
Figure 14. Variation of conductivity with fumed silica concentration for nano-dispersed polymer gel electrolytes containing 10wt% PMMA in solution of 1M benzoic acid in DMA.
The presence of second conductivity maxima at ~8wt% of fumed silica in Figure 12 and 13 is due to the interlocking of fumed silica particles within the PMMA chains which increases viscosity along with promoting composite nature and as a result the second maxima is due to the formation of high conducting interfacial layer between the particles of fumed silica and PMMA gel [32]. As the grain size of fumed silica is quite small (7nm) so maxima is observed at low concentrations of fumed silica whereas in the case of micron size Al2O3 the maxima is generally observed at higher concentrations (20-30wt%) of insulating materials in the composite polymer electrolytes.
The enhanced dissociation of o-OHBA with the addition of fumed silica at low concentrations was also examined by pH measurements. The variation of pH with the concentration of fumed silica for nano dispersed polymer gel electrolytes containing 4 and 10wt% PMMA in 0.5 and 1M o-OHBA solutions in DMA are given in Figure 15. Similar behaviour has been observed in each case. pH has been found to decrease with the initial addition of fumed silica and then reaches a saturation value. The decrease in pH indicates an increase in acidity which is due to an increase in free H+ ion concentration due to the enhanced dissociation of undissociated acid as proposed above while explaining conductivity results. In Figure15, the decrease in pH of polymer gel electrolytes containing 10wt% PMMA is less than those containing 4wt% PMMA which could be due to higher viscosity of polymer gel electrolytes containing 10wt% PMMA. The nearly constant value of pH above around 2wt% fumed silica suggests that the second maxima observed at ~8 wt% fumed silica is not related to a change in free H+ ion concentration.
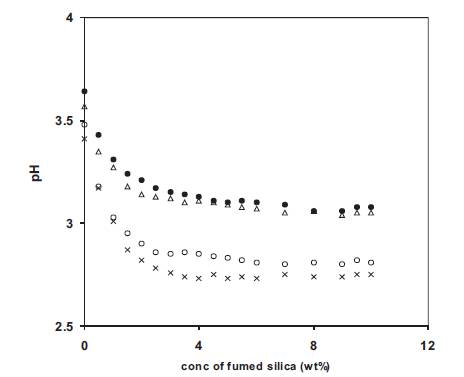
Figure 15. Change of pH with fumed silica concentration for nano-dispersed polymer gel electrolytes containing 4 (o) and 10 (•) wt% PMMA in solution of 0.5M o-hydroxy benzoic acid and for electrolytes containing 4 (x) and 10 (Δ) wt% PMMA in solution of 1M o-hydroxy benzoic acid in DMA
The addition of fumed silica also results in a large increase in viscosity and viscosity of nano dispersed polymer gel electrolytes was also measured as a function of the concentration of fumed silica and the results are given in Figure16 and 17 for electrolytes containing 4 and 10wt% PMMA and 0.5 and 1M o-OHBA. From the results, it has been observed that the viscosity of electrolytes containing 10wt% PMMA is higher than those containing 4wt% PMMA at all concentrations of fumed silica. The viscosity of electrolytes containing 1M o-OHBA is higher than those containing 0.5M o-OHBA at all concentrations of fumed silica. The viscosity of different nano–dispersed polymer gel electrolytes at low fumed silica concentrations is lower than at high fumed silica concentrations. The change in slope observed in Figure 17 indicates higher viscosity at high fumed silica concentrations which plays a dominant role in conductivity modification and the decrease in conductivity above 8wt% fumed silica is due to this reason.
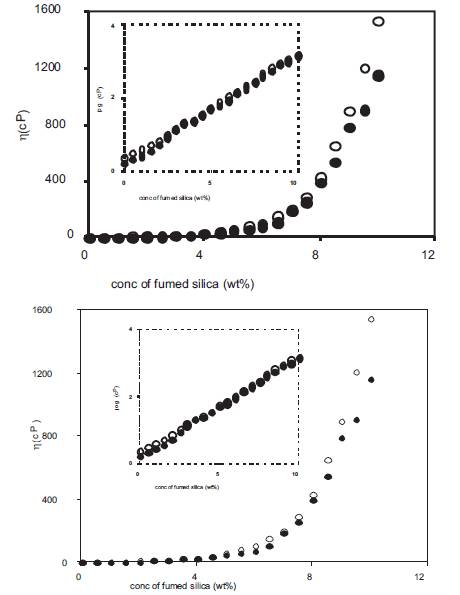
Figure 16. Viscosity of nano dispersed polymer gel electrolytes containing 4wt% PMMA in solution of 0.5M (•), 1M(o) o-hydroxy benzoic acid in DMA as a function of fumed silica concentration.

Figure 17. Viscosity of nano dispersed polymer gel electrolytes containing 10wt% PMMA in solution of 0.5M (•), 1M (o) o-hydroxy benzoic acid in DMA as a function of fumed silica concentration.
The conductivity of polymer gel electrolytes containing 10wt% PMMA in the 0.5M solution of o-OHBA in DMA and the corresponding nano dispersed polymer gel electrolytes obtained by adding 10wt% fumed silica was measured as a function of temperature and the variation of log conductivity vs reciprocal temperature is given in Figure18. The conductivity of nano dispersed gels is higher than the corresponding gel electrolytes at all temperatures and the change in conductivity over the operational range of temperature is very small which is desirable for their use in various devices. For a good electrolyte, the conductivity should not change with time and this was studied by measuring the conductivity of nano dispersed gel electrolytes as a function of time over a period of three weeks and the results are given in Figure19. There is no appreciable change in the conductivity of nano dispersed gel electrolytes for this period.
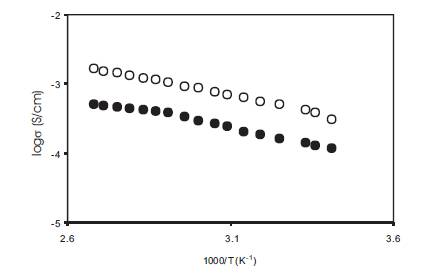
Figure18. Conductivity as a function of temperature for gel electrolytes (•) containing 10wt% PMMA in solution of 1M o-hydroxy benzoic acid in DMA and nano dispersed gel electrolyte (o) containing 10wt% fumed silica.
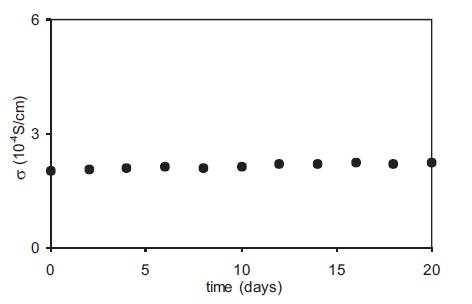
Figure 19. Variation of conductivity of nano dispersed gel electrolytes (DMA + 1M o-hydroxy benzoic acid + 10wt% PMMA + 10wt% fumed silica) as a function of time.
From the above studies, it has been observed that the nano dispersed gels obtained by adding nano sized fumed silica to proton conducting polymer gel electrolytes containing a weak acid (o-hydroxy benzoic acid) show o high conductivity (10-4 S/cm) at 25o C along with high viscosity (~105 cP). The conductivity remains constant with time and there is only a small change over the operational range of temperature. Thus these nano dispersed gel electrolytes are suitable for use as electrolytes in polymer electrolyte membrane fuel cells and other devices over a wide temperature range. However their compatibility with electrodes etc. have to be checked before their actual use.
Nano dispersed polymer gel electrolytes with conductivity of 10-4 S/cm at 25o C along with good dimensional stability have been obtained. The enhanced dissociation of ohydroxy benzoic acid with the addition of PMMA monitored by pH measurements results in an increase in conductivity and s(gel) > s(liquid) have been observed. The increase in conductivity and viscosity with polymer addition has been found to depend upon the molecular weight of the polymer used. The addition of nano sized fumed silica to polymer gel electrolytes also results in further conductivity enhancement along with an improvement in mechanical strength. The increase in conductivity at low fumed silica concentration has been explained to be due to the enhanced dissociation of o-hydroxy benzoic acid whereas at high fumed silica concentrations it is due to the formation of a high conductivity interfacial region between the particles of fumed silica and polymer gel electrolytes. The change in conductivity with temperature is very small and no change in conductivity has been observed with time which is desirable for the applications of nano dispersed gels in fuel cells and other devices.
The author is thankful to University Grants Commission, New Delhi for financial support in the form of research scheme No. 8-2(172)/2011 (MRP/NRCB).