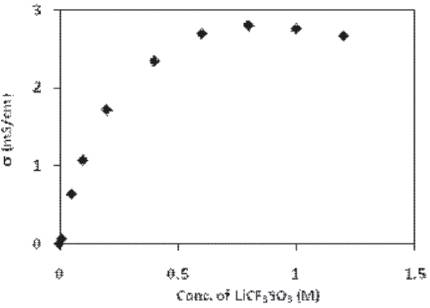
Figure 1. Variation of conductivity of liquid electrolyte (PC– xM LiC3F SO3 ) with the concentration of LiCF3 SO3 in PC.
Effect of polymer (PMMA) and salt (LiCF3SO3 ) on the conducting behaviour of polymer gel electrolytes in non-volatile solvent (PC) has been studied. The maximum ionic conductivity ( = 2.92 mS/cm) of polymer gel electrolyte has been observed at room temperature. An anomalous conducting behaviour is observed at small content of PMMA addition in gel electrolyte. Small increase in conductivity observed with increasing temperature (range 10o -70o C) is in factor-wise only, which makes these gel electrolytes suitable for many device applications.
Polymer gel electrolytes are materials of current research interest due to their high ionic conductivity (> 10-4 S/cm) comparable with polymer electrolyte. Generally, polymer gel electrolytes belong to the salt-solvent-polymer hybrid system and are prepared by immobilizing the salt solution with a suitable polymer matrix. Salt provides ions in solvent, whereas solvent helps to solvate the salt retained in the electrolyte matrix and polymer provides mechanical stability to gel electrolyte [1-4]. Initial work on the gel electrolytes were mainly with lithium salts since 1973 due to the small radii of lithium ion, high ionic conductivity and their use in different solid state ionic devices viz. high density batteries, electrochromic windows, supercapacitors, sensors etc. [5,6]. The conductivity (σ) of electrolyte is generally expressed in terms of the charge concentration (n) and the mobility (μ) of the ions as σ = nqμ, where q is the charge on the mobile species. Polyvinylidenefluoride (PVdF), polymethylmethacrylate (PMMA), polyethylene oxide (PEO), polyvinylidenefluoride-hexafluoropropylene (PVdF-HFP) etc. are some of the polymers commonly used in electrolyte for their jellification [7-9].
In the present research work, PMMA has been used in the synthesis of polymer gel electrolytes containing LiCF3 SO3 as a salt and propylene carbonate (PC) as a solvent. The effect of smaller and higher content of polymer addition on the conductivity behaviour of gel electrolyte has also been studied. Variation of conductivity has also been observed with an increase in the temperature of polymer gel electrolyte containing LiCF3 SO3 in PC.
Polymethylmethacrylate (PMMA) (Aldrich, Mw = 1,20,000), lithium trifluoromethane sufonate (LiCF3SO3 ) (Aldrich) and propylene carbonate (PC) (Aldrich) were used as the starting materials for the preparation of liquid and gel electrolytes. At room temperature, LiCF3 SO3in different concentrations (of molarities values) was dissolved in the solvent (PC) to obtain liquid electrolyte. The gradual addition of PMMA along with continuous stirring at 40o C results in the formation of gel electrolyte. Ionic conductivity of liquid and gel electrolyte was measured with the help of conductivity meter (WTW 3210), which is based upon the four probe method and inbuilt temperature sensor.
Variation of ionic conductivity of liquid electrolyte by dissolving LiCF3 SO3 salt (in different molar ratios) in PC has been observed and is shown in Figure 1. The conductivity increases linearly with smaller additions of LiCF3 SO3 , reaches a maximum value and shows a decrease with further addition of salt. At low salt concentrations, salt upon dissociation in liquid electrolyte provides free ions, which results in an increase in conductivity and the maximum ionic conductivity obtained is 2.81 mS/cm for liquid electrolyte at 0.8 molar concentration of salt.

Figure 1. Variation of conductivity of liquid electrolyte (PC– xM LiC3F SO3 ) with the concentration of LiCF3 SO3 in PC.
But at the higher salt concentration, there is a small decrease in conductivity, which may be explained to be due to the formation of ion aggregates.
Gel electrolyte was prepared by adding polymer (PMMA) to liquid electrolyte and results obtained are shown in Figure 2. It has been observed that the conductivity of gel electrolyte containing 1M LiCF3 SO3increases with the addition of PMMA and reaches a maximum value of 2.92 mS/cm at 0.5 wt. % of PMMA and then decreases to a value of 2.04 mS/cm at 10 wt. % of PMMA. An increase in conductivity of gel electrolyte with small content of polymer may be explained with that the polymer chain is assumed to breathe in and out while it opens or folds occupying different volume in the process, which leads to localized pressure fluctuations assisting either in separating the neutral ion-associated pairs or ''loosen up'' of the viscosity controlled mobility or both resulting in the conductivity increment [10]. This fact has also been observed in our results and is shown in Figure.2. In 1999, Grillone et al have observed an increase in the ionic conductivity on jellification of gel electrolyte containing salicylic acid and in 2000, Chandra et al. have suggested a “breathing polymeric chain model” to explain the interaction of polymer with the liquid and gel electrolyte [10,11]. But, however, the decrease in conductivity is small and by a factor only. A small decrease in conductivity at higher polymer content suggests that the polymer acts as a stiffener, resulting in an increase in viscosity which tends to decrease mobility and hence conductivity.
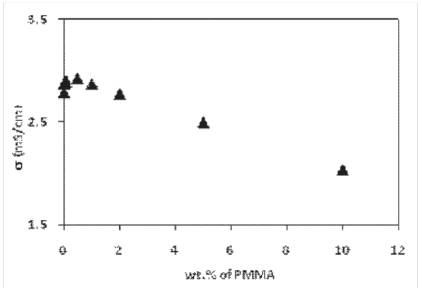
Figure 2. Change of conductivity of polymer gel electrolyte at different content of polymer.
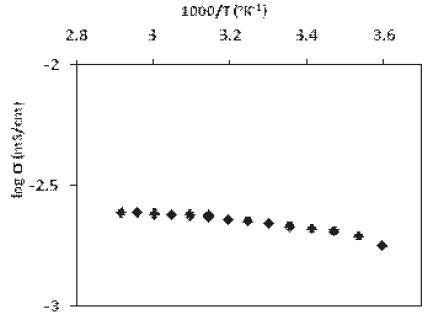
Figure 3. Variation of conductivity of polymer gel electrolyte as a function of temperature.
Conductivity of PMMA based gel electrolyte increases with increase in temperature and this behavior follows a good agreement with the theoretical relation σ = σ0 exp(-β/T), where β-adjustable parameter, σo–constant, T- temperature. Also, the increase in conductivity of gel electrolyte with increase in temperature is small and factor wise only, which makes it suitable for many device applications [12].
Lithium ion conducting PMMA based polymer gel electrolyte having maximum ionic conductivity at room temperature is 2.92 mS/cm. A small increase in the conductivity of gel electrolyte with lower content of polymer has already been explained with “Breathing Polymeric Chain Model” and small decrease in the conductivity indicates that polymer acts as a stiffener only at higher content of polymer. Small increase in the conductivity of gel electrolyte with increase in temperature makes them suitable for many device applications.
Authors are highly thankful to Dr. K.N. Kaul, Principal, D.A.V. College, Amritsar for helping to carry out the experimental work, generous support and valuable suggestions.