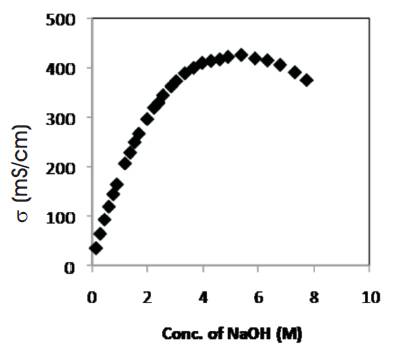
Figure 1. Variation of room temperature conductivity of liquid electrolyte with salt concentration.
Ionic conductivity of xanthan gum based gel electrolytes containing NaOH have been studied and the maximum ionic conductivity (σ = 88.8 mS/cm) at room temperature has been recorded. The behavior of ionic conductivity with the rise in temperature has been observed. A small change in the ionic conductivity of xanthan gum based gel electrolytes containing sodium hydroxide (NaOH) has been observed with the passage of time. pH value of gel electrolyte containing NaOH shows that they are basic in nature.
A Gel is defined as substantially dilute cross linked system and is categorized principally as weak or strong (in terms of less or more viscous) depending upon their flow behavior in steady state [Ferry, 1980] [1]. Gel electrolyte is the hybrid mixture of salt, solvent and polymer, in which salt provide ions upon it's dissociation in solvent and creates conducting medium. The solvent’s property is to dissolve the salt; whereas polymer increases the mechanical stability of the electrolyte [2-4].
Number of protons conducting polymer gel electrolytes have been studied since last two decades and categorized as: strong acid based gel electrolytes, weak acid based gel electrolytes and natural gum based gel electrolytes [4]. Strong acid (like HCl, H3PO4 and H2SO4 etc.) based gel electrolytes are mostly not suitable for a device application due to their corrosive nature that results in the degradation of the gel electrolytes. Whereas, weak acid based gel electrolytes are materials of current interest due to their high ionic conductivity at room temperature and also non-degradable nature.
Also natural gums consist of polysaccharides which are extracted from trees and plants, they are materials of high molecular weight, which are mostly soluble in water and provide high ionic conductivity and also capable of large increase in liquid viscosity even at small concentrations. They are also used as adhesives, binding agents, encapsulating agents, flocculating agents, swelling agents, foam stabilizers, etc. [5-7].
In the present research work, the ionic conductivity and pH of xanthan gum based gel electrolytes containing NaOH have been reported.
Xanthan gum is a polysaccharide secreted by the bacterium Xanthomonas campestris, mainly composed of pentasaccharide repeat units containing glucose, sucrose and lactose and is produced by the fermentation of glucose, sucrose and lactose.
Xanthan gum based gel electrolyte was prepared by dissolving salt (NaOH) in distilled water to prepare liquid electrolyte (NaOH solution). After that an appropriate amount of xanthan gum is kept (Aldrich) in NaOH solution; with continuous stirring and keeping it for 24 hours at room temperature, a homogeneous gel electrolyte in viscous form was obtained.
The extent of ion transport in gel electrolyte represents it's ionic conductivity i.e. σ = nqμ, where n represents the number of charge carriers, be the mobility of ions and q be the charge on the ion [8, 9]. The ionic conductivity of gel electrolyte was measured with conductivity meter (WTW 3210), which is based upon four probe method. To check the acidic/basic behavior of gel electrolyte, pH measurement was done with the help of pH meter.
Xanthan gum is highly soluble in both cold and hot water and this behaviour is related with the polyelectrolyte nature of the xanthan molecule. Due to their special properties, xanthan gum is useful in many industrial applications, especially in the food industry, as well as it is used as a thickener to stabilize suspensions and emulsions [10].
Lonic conductivity of liquid electrolyte (solvent: distilled water) has been studied as a function of salt (NaOH) concentration at room temperature and is as shown in Figure1.
The conductivity of solvent (distilled water - DW) (~10-6 S/cm) increases by four order of magnitude with the addition of small concentration of NaOH salt, which may be explained to be due to the dissociation of salt into ions in distilled water resulting on an increase in the number of charge carriers and hence conductivity value increases. As the ionic conductivity is given by σ=nqμ, where n represents the number of charge carriers, μ be the mobility of ions and q be the charge on ion. The mobility of ions is given by μ=q/6πrη. Here η is the viscosity of electrolyte, q is the charge on the ion and r is radius of carrier ion.
From Figure 1, it has also been observed that conductivity increases linearly (upto 270 ms/cm) with the increase of salt (NaOH) concentration in distilled water, which has already been explained and is due to the dissociation of salt (NaOH) into ions (Na+ & OH- ) and at higher salt concentration, a small decrease in conductivity value has been recorded, which may be due to the formation of ion aggregates along with the increase in viscosity of liquid electrolyte.

Figure 1. Variation of room temperature conductivity of liquid electrolyte with salt concentration.
The conductivity of liquid and gel electrolyte has been studied with increase in temperature and the results are shown in Figure 2. It has been observed that the conductivity of liquid and gel electrolyte decreases with increase in temperature, which is in contradiction to the T theoretical explanation {i.e. σ = σ0 exp(-E0 /KBT )}. This may be due to the evaporation of water on raising the temperature of gel electrolyte, which tends to increase the restriction of ions in liquid as well as gel matrix, leading to the decrease in mobility of ions and hence conductivity and this type of explanation has already been explained by Karmann in 1981 [11]. Also the decrease in conductivity with increase in temperature is small which may make the suitability of these gel electrolytes for device application, particularly for fuel cell application.
It has been further observed that the conductivity of xanthan gum based gel electrolyte is lower than that of liquid electrolyte at all temperature regions. This may be explained to be due to the higher viscosity of gel electrolyte as compared to that of liquid electrolyte with the addition of xanthan gum.
The conductivity of gel electrolyte has also been studied at different time span and the results are shown in Figure 3. The conductivity of xanthan gum based gel electrolyte containing NaOH increases upto 20 days and then decreases with the passage of time. This may be explained due to the continuous movement and interactions of OH- , H+ , H3O+ , Na+ ions in gel electrolyte with the passage of time [12]. As there is not much change in conductivity and gel electrolyte has been observed fungus free even after 50 days, this indicates their suitable candidature for fuel cell application.
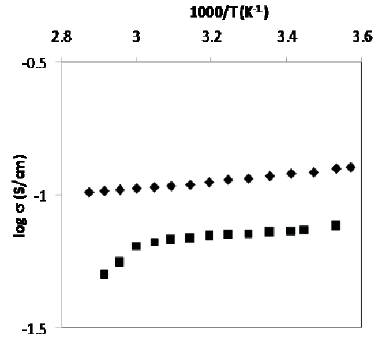
Figure 2. Conductivity variation of liquid ( ) and
gel (
) and
gel ( ) electrolyte with temperature
) electrolyte with temperature
pH value of xanthan gum based gel electrolyte containing sodium salt was studied with the passage of time and it's observed values for different gel electrolytes lies between 12 - 14. This shows that these gel electrolytes are basic in nature.
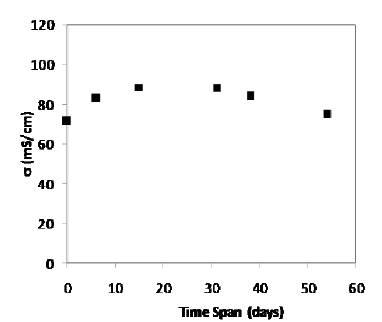
Figure 3. Variation of ionic conductivity of gel electrolyte with the passage of time at room temperature.
The ionic conductivity of xanthan gum based gel electrolyte containing sodium hydroxide is lower than the liquid electrolyte. Also the conductivity of the gel electrolyte decreases with increase in temperature. Conductivity of gel electrolyte variation with passage of time is small and is factor-wise only, which may make it suitable for fuel cell application. pH value of gel electrolyte shows their basic behaviour.
The author would like to pay thanks to Dr. K.N. Kaul, Principal, DAV College, Amritsar for providing the necessary infrastructure at the work place.