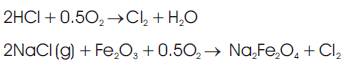
Fireside corrosion of boiler tubes in bio medical waste incinerator plants has been of great concern for plant operators, boiler designers, and boiler tube manufacturers since quite a few number of boiler tubes in these plants suffered from wall thinning due to hot corrosion. The corrosion problem at high temperature is more serious due to the highly aggressive environment in bio medical waste incinerator plants such as Cl2 , HCl, alkali metals and heavy metals. Corrosion resistant coatings are the key technologies to reduce the maintenance in bio medical incinerator plants by reducing the hot corrosion problem. Different corrosion resistant coatings processes have been developed such as thermal spray, HVOF, HVSFS, PVD and cold spray. Employing the corrosion resistant coatings ensure the high corrosion and wear resistant of the boiler tubes. This paper highlights the hot corrosion in bio medical incinerator plants, its effects and its protection with corrosion resistant coatings. Cold spray coatings have been reviewed with the aim of summarizing their high temperature corrosion resistance properties.
Bio Medical wastes are considered as hazardous waste because they contain toxic materials, infectious, or noninfectious wastes and they are considered as hazard to millions of patients, health care workers, and visitors. Treatment processes for medical wastes is incineration. Incineration is a thermal process, which destroys most of the waste including microorganisms. In India there are 157 biomedical incinerators out of which149 are operating and 8 are under installation. The proper combustion temperature for medical waste incineration ranges from 900- 1200°C by Labib (2005). More than 3.5 million of medical waste is generated annually within United States. On the other hand, since the first and the second energy crisis took place in 1973 and 1979, respectively, efficient development and management of energy resource in industry has become a noticeable issue. It is known that incineration involves a high-temperature combustion process in which it represents an exothermic reaction in nature. In other words, after the destruction of most combustible or organic components in waste, a large amount of by-product, viz., heat, will be generated. For a bio medical waste incinerator the released energy is commonly used to produce steam or generate electricity. The revenues received from the sale of steam or electricity can partially offset the high operating and maintaining costs of an incinerator by Chen et al. (2001). But fireside corrosion of boiler tubes in incinerators has been of great concern for plant operators, boiler designers, and boiler tube manufacturers since quite a few number of boiler tubes in these plants suffered from wall thinning due to corrosion by Otsuka (2008). The condition in the boilers of power generators at waste incineration plants contribute to severe corrosion of furnace wall tubes. This corrosion results in the cost and inconvenience of replacing boiler tubes as often as every 3 or 4 years by Matsubara et al. (2007). The corrosion problem at this high temperature (900- 1200 °C) is more serious due to the highly aggressive environment and known as hot corrosion. Hot corrosion is the accelerated oxidation of materials covered with a thin film of fused salt exposed to an oxidizing gas atmosphere at elevated temperatures by Zenga & Lia (2005).
Degradation of components in hot sections of incinerators are mainly due to the high temperature oxidation, hot corrosion and erosion. Superalloys have been developed for high temperature applications, but they are not able to meet the requirements of both the high-temperature strength and the high-temperature erosion–corrosion resistance simultaneously by Kamala et al. (2007).
Corrosion is both costly and dangerous. Besides indirect costs due to shutdown and loss of efficiency, billions of dollars are spent annually for the replacement of corroded structures, machinery and equipment, and their premature failure can result in human injury or even loss of life. Metallic corrosion costs about $300 billion per year in United States, which is approximately 3.1% of the nation's Gross Domestic Product (GDP) by Koch et al. (2002). It is important to understand the nature of all types of environmental degradation of metals and alloys as vividly as possible so that preventive measures against metal loss and failures can be economically devised to ensure safety and reliability in the use of metallic components by Sidhu et al. (2006). Therefore, in this paper more emphasize is given to understand the degradation of materials by hot corrosion in bio medical waste incinerator and its protection with thermal spray coatings.
Incineration has been used as method for processing waste since the beginning of the century. Incineration is a thermal process, which destroys most of the waste including microorganisms. Over the past few decades it has evolved into a widely used, established technology with reliable modern facilities operating on a fully commercial basis in some advanced countries. Modern incineration plants are now almost always built with energy recovery and heat is recovered to provide steam or hot water for industrial or domestic users, or for electricity generation.
Treatment processes for medical wastes comprise autoclaving, microwaving, chemical disinfection, radiation, plasma system, and incineration. Incineration is a thermal process, which destroys most of the waste including microorganisms. Combustion process must be under controlled conditions to convert wastes containing hazardous materials into mineral residues and gases. Medical waste incinerators may emit a number of pollutants such as particulate matter, acid gases, toxic metals, and toxic organic compounds products of incomplete combustion, e.g., dioxins, furans, and carbon monoxide, as well as sulfur oxides and nitrogen oxides. So, there should be a reduction of emissions of most of these pollutants by air pollution control devices by Labib (2005).
The common objectives of waste incineration are volume reduction, removal of volatile; combustible; and destruction of toxic and pathogenic materials under the combustion conditions. The combustion conditions are: adequate free oxygen to be available in the combustion zone, turbulence, proper combustion temperature (900- 1200 °C), a long residence time of the waste gases in a hot oxidizing environment (>2 or 3 seconds) by Labib (2005). Also a suitable site for hazardous waste incinerator would be a site where the resulting air emissions would not diminish the air quality for the residents of a city or town.
As proper combustion temperature (900- 1200 °C), so cofired (such as diesel fired) incinerator are used for medical waste incineration. A lot of heat energy is available at this high temperature which can be used to provide steam or hot water for industrial or domestic users, or for electricity generation.
Hot corrosion has been observed in boilers, internal combustion engines, gas turbines, fluidized bed combustion and industrial waste incinerators since 1940s. However, it became a topic of importance and popular interest in late 1960s when gas turbine engines in military aircraft suffered severe corrosion during the Vietnam conflict while operating over sea water (Rapp, 1986; Rapp, 1994). The first technical publication on hot corrosion was by Simons et al. They outlined a reaction mechanism involving metal sulfidation by Na2 SO4 with emphasis on the accelerated oxidation of a sulfide-base eutectic by Sidhu et al. (2005).
Hot corrosion has become a significant degradation mechanism in incinerator boiler tube materials due to high operating temperatures and operation in environments containing alkali metal salts, especially in medical waste incineration environment.
A metal or an alloy resistant to oxidation is determined by the properties of the oxide it forms when it is exposed to a certain environment. The formed oxide functions as a barrier against further oxidation, as electrons, oxygen and metal ions need to be transported through the oxide in order for the oxidation process to proceed. This is especially important in the field of high temperature corrosion. Typical oxides that are considered to be protective at elevated temperatures are Cr2O3 (Eskolaite) and Al2O3 (Corundum), this is due to their low ionic and electronic conductivity. Cr2O3 (s) normally forms on commercial Fe–Cr and Fe–Cr–Ni alloys (referred as stainless steels), and a- Al2O3 (s) on Fe–Cr–Al alloys. However, Fe is the main constituent of these alloys, which means that Cr and Al need to be selectively oxidized. Furthermore, the chemical integrity of the protective oxide needs to be maintained over time ( Asteman & Spiegel, 2008).
Corrosion resistant alloys depend upon selective oxidation to form the dense, compact protective scales of Cr2O3 and Al2O3 for the resistance. During hot corrosion a degradation sequence consisting of the eventual displacement of a more protective reaction product barrier by a less protective product is followed. The hot corrosion degradation sequence is not clearly evident, and the time for which protective scale is stable is influenced by a number of factors such as alloy composition, fabrication condition, gas composition and velocity, salt composition, salt deposition rate, temperature, temperature cycle, erosion and specimen geometry which effects the initiation of hot corrosion. The propagation modes of hot corrosion which are related to the reaction between the molten deposits and alloy can be of two types: 1) a non protective reaction product is formed because of fluxing action of molten deposit and 2) a component of the deposit like S or Cl plays a dominant role in the process of forming a non protective reaction product ( Wang, 1996).
During combustion in medical waste incinerator, considerable quantities of alkali chlorides are formed in the chamber, mainly potassium chloride (KCl) and sodium chloride (NaCl), due to the fact that K, Na and Cl are relatively volatile elements, which results in the enrichment of chlorine in the flue gas. Fine fly ash particles in flue gas with high chlorine content tend to form corrosive deposits on the surface of tubes. The presence of chlorine and alkali chloride in the deposit is expected to play a dominate role in the mechanism of selective chlorine corrosion ( Michelsen et al., 1998).
Metals corrode at high temperatures in the absence of liquid electrolyte, which is required in the case of low temperature aqueous corrosion. The mechanism for corrosion is determined by the most abundant deposits observed on the metal after corrosion, i.e. oxidation by metal oxides, sulfidation by metal sulfides, sulfidation/ oxidation by mixtures of sulfides and oxides, carburization by metal carbides, and chlorination of metals to metal chlorides. In general there are two principal mechanisms of high temperature corrosion ( Albina, 2005):
This mechanism occurs at metal temperatures above 450o C and comprises several steps:
1. Oxidation of hydrogen chloride in gas with water vapor or reaction of chlorides such as NaCl with metal oxides forms chlorine (Cl2 ) at the tube surfaces:
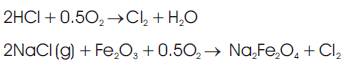
2. Penetration of chlorine through the metal oxide scale to the oxide/metal interface and reaction with iron or other metal component of the tube to form metal chlorides:
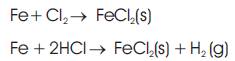
3. Diffusion of metal chloride vapor outward through the scale covering the tube and reaction of the vapor with oxygen in the gaseous layer surrounding the tube to form metal oxide and chlorine:
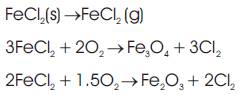
In step (3), chlorine is released and gets diffused to bulk gas. However, part of the liberated chlorine may diffuse back to the oxide/metal interface and react with the metal and form volatile metal chlorides again. Therefore, a cycle is formed that provides a continuous transport of metal, in the form of chloride, away from the surface towards the higher oxygen partial pressure
When volatilized chloride salts in combustion gases come into contact with the cooler tube surface they condense and form either liquid or solid deposits that may contain sulfates and alkali chlorides. Deposited metal chlorides react with gaseous SO2 or SO3 to form condensed alkali sulfates.
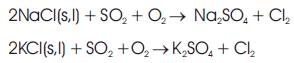
The deposits contribute to corrosion in two ways: (a) the chlorinated species in the deposit cause a reaction similar to the gas phase active oxidation described above; (b) the presence of chlorides in the deposits may result in the formation of low melting point eutectics (i.e., salt solutions characterized by lowest possible melting point) which may dissolve the oxide layer that is protecting the metal surface.
Hot corrosion is considered as a major failure mode for high temperature components of many important engineering systems such as aircraft, land-base gas turbines and boiler tubes of incinerator. This accelerated attack results from condensation of films containing molten salts, such as sulfates, chlorides, or vanadates.
Fireside corrosion of boiler tubes in medical waste incinerators is a very serious problem. Several remedial measures regarding materials selection, boiler design, and operational conditions have been proposed and some are successfully applied in practice, but still the superheater steam temperature of major waste-heat boilers is limited below 450oC (Yukawa & Bousei-Kanri 1995), which is indeed considerably lower than other types of conventional boilers firing coal, oil, etc.
Hot corrosion problem is so severe in bio medical waste incinerator in three bio medical incinerator plants visited by the author in Punjab, India. None of these plants use the heat energy released during incineration because of huge cost involved in replacing the tubes due to degradation by hot corrosion. In 2004, the Waste-To-Energy Research and Technology Council (WTERT) conducted a corrosion survey of several Waste to Energy facilities in United States One of the results of the survey showed that the non-scheduled downtime due to corrosion ranged from 0 to 20 days per year. Another result showed that the yearly maintenance cost per boiler unit due to corrosion ranged from $18,000 to $1,200,000 the maintenance cost due to corrosion ranged from $0.23 to $8.17 per ton of Municipal Solid Waste combusted. The typical cost is in the range of $4 per short ton of Municipal Solid Waste combusted. The capital cost and maintenance cost accounts approximately 60% and 15% of the yearly cost of a Waste to Energy facility, respectively in German ( Zwahr, 2003). Therefore, the corrosion problems will cost Waste to Energy approximately 5% of its yearly total cost, if the corrosion/total maintenance cost ratio of 1/3 applies. The actual cost will be even higher if the revenue loss due to shutdowns because of corrosion is taken into account ( Lee et al., 2007).
Flue gas temperature, along with flue gas velocity, etc., must be an important corrosion factor to affect corrosion as well ( Licata et al., 1992), but quantitative explanation relating these factors to the corrosion of boiler tubes does not seem to have been presented yet. Corrosion rate of boiler tubes obtained in several field tests does have clear correlation with the deposit chemistry (Report, 2000).
Over several years of operating experience, the Waste to Energy industry has developed general approaches to reduce corrosion that can be classified as primary and secondary measures. Primary measures seek to eliminate corrosion by influencing the process conditions in the boiler. Some of these methods include : (a) improvement of process control, in particular minimizing fluctuations in gas temperature; and (b) design modifications, such as process gas recirculation to alter flow dynamics, enhancing mixing of gas through gas recirculation, and design of the boiler system (e.g. horizontal vs. vertical boiler). Secondary methods of protection are applied to extend the lifespan of the boiler tubes. In the past ten years, many kinds of corrosion-resistant material systems have been tested and applied to actual boilers. For example, high Cr-high Mo nickel-base alloys and high Cr-high Si ferronickel alloy tubing products are used in Waste to Energy plants. Further more, coating systems such as High Velocity Oxygen Fuel (HVOF) thermal spray and weld overlay has been developed and applied to advanced Waste to Energy boilers. Other measures include a new technique named ''Targeted In- Furnace Injection'' which makes the ash deposits more friable by means of injecting chemicals, such as MgO, directly into the combustion chamber, and the extensive use of high conductivity refractory lining and ceramic tiles in the lower half or the entire height of the combustion chamber. Some of these methods require either the retrofit of existing WTE boilers or the redesign of boilers of new Waste to Energy facilities. In the case of retrofits, the construction time for making equipment changes is an very important parameter since the required shutdown can affect the economic viability of the WTE facility ( Zwahr, 2003).
Corrosion damage is a major issue in waste incineration plants: due to repair work and involved standstill it is highly expensive. So materials used in high-temperature environments, oxidation and hot corrosion resistance are key issues ( Lin et al., 2007).
Moreover increasing demand for more electricity, reduced plant emissions and greater efficiency is forcing power plants to increase the steam temperature and pressure of boilers. Ultra-supercritical steam conditions greater than 31MPa and 600°C have been adopted, and the thermal efficiency of a pulverized coal-fired boiler has been obtained up to 45%. Superheater and re-heater materials will therefore be required which have high creep rupture strength and high corrosion resistance at temperatures of about 750°C and above ( Blum, 1997; Evans et al., 2004). Superalloy can be used to meet these stringent material targets ( Smith et al., 1999), but they are unable to meet both the high-temperature strength and the high temperature corrosion resistance requirements simultaneously ( Goebel et al., 1973; Sidhu et al., 2005). Protective coatings can be used on superalloys to meet the latter requirement. Coatings can add value to products up to 10 times the cost of the coating ( Matthews et al., 1998 ). Even if the material withstands high temperature without a coating, the coating enhances the lifetime of the material. Protective surface treatments are widely used at low temperature, the use of these at elevated temperature is more recent ( Stroosnijder et al., 1994; Yoshiba, 1993). The demand for protective coatings has increased recently even for almost all types of superalloys, since hightemperature corrosion problem has become much more significant for these alloys with increasing operating temperatures of boilers, turbines and heat engines. The necessities for higher performance and increased efficiency has resulted in the progressive increase in their operation temperatures ( Stott et al., 1994; Conner et al., 1994 ). As a result, components operating at high temperature within such plants are coated or surface treated ( Nicholls, 2000; Liu et al., 2001).
Corrosion resistant coating has a great demand to withstand hostile environments in different heat engines and heat exchangers used in industries ( Datta, 2001). There they are subjected to combined attack of high temperatures and condensed phases such as Na2SO4 and NaCl. Deposition of these salts on the hot zone components leads to severe hot corrosion resulting in accelerated deterioration of the structural materials ( Liu et al., 2005 ). Further, to increase the efficiency of these heat engines, efforts are being made to increase the operating temperature and the non-availability of ideal material has become a serious limitation. Temperatures associated with hot corrosion degrade the material at a very rapid rate and load carrying ability of the component decreases leading eventually to catastrophic failure ( Eliaz et al., 2002). Therefore, an effective surface coating is often applied on these components to protect them from different aggressive environments. Generally, diffusion coating, overlay coating, thermal barrier coating and functionally graded overlayer coating are designed for the surface protection of the substrate material from high temperature hot corrosion ( Das et al., 2008).
Thermally-sprayed protective coatings find numerous industrial applications in chemical and petrochemical plants, paper processing rolls, flexographic equipment, components for packaging machines, pump parts (impellers, seals, body, journal bearings), hydraulic machinery (pistons, rods), coastal and off-shore installations, etc ( Davis, 2004). In many of these applications, the coatings are required to protect the substrate from corrosive environments. They must not therefore have any interconnected porosity, otherwise galvanic corrosion of the substrate would occur, since the coating materials suitable for operation in severe conditions (Ni- and Co-based alloys and cermets) are electrochemically more noble than ordinary steel substrates ( Bolelli et al., 2008). There are mainly three kinds of protective coatings, including diffusion aluminide coating, MCrAlY overlay coating and thermal barrier coating, which can be used on nickel-based superalloys at present ( Rena et al., 2005; Gurrappa, 2000). Thermal spray coatings are economical, it can be produced by means of relatively simple techniques and offer excellent corrosion and wear protection. So these coatings have found use in various industrial applications. One of the advantage of thermal spray coatings is the fact that the molten or partly molten coating material droplets deposit on the substrate material without melting. So very less temperature rise of the substrate material takes place during thermal spray coating. Therefore no influence on the heat treatment, chemical composition of substrate material is observed in thermal spray coating.
There are many coating deposition techniques available, and choosing the best process depends on the functional requirements, adaptability of the coating material to the technique intended, level of adhesion required (size, shape, and metallurgy of the substrate), availability and cost of the equipment ( Bhushan & Gupta,1991). Coatings technique can be classified as shown in Figure 1.
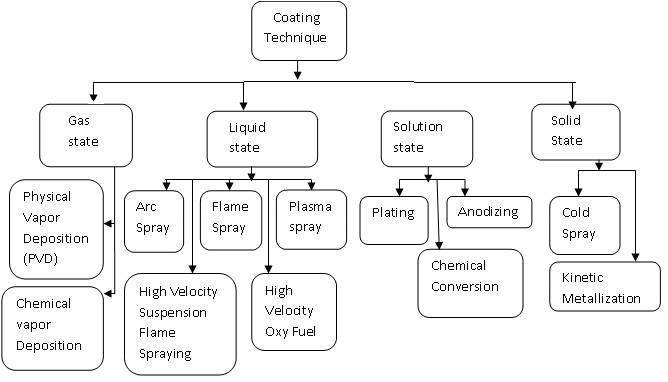
Figure 1. Different Coating Techniques.
Among the various coating methods, cold spraying is a new emerging coating technique. Cold gas dynamic spray, simply called cold spraying, is a rapidly developing technology for the preparation of coatings or bulk materials in the solid state. Cold spray processing was developed in the former Soviet Union more than a decade ago as an offshoot of supersonic wind tunnel testing ( Davis, 2004; Papyrin et al., 2007) . There are different approaches known by different names such as: Cold Gas Dynamic Spraying, Kinetic Spraying, High Velocity Particle Consolidation (HVPC), High Velocity Powder Deposition and Supersonic Particle/Powder Deposition (SPD). The basic principle of the cold spray process is very simple as shown in Figure 2. A high velocity (300 to 1200 m/s) gas jet, formed using a deLaval or similar converging/diverging nozzle, is used to accelerate powder particles (1 to 50μm) and spray them onto a substrate, located approximately 25 mm from the exit of the nozzle. where they impact and form a coating. The kinetic energy of the particles rather than high temperature helps these particles to plastically deform on impact and form splats, which bond together to produce coatings and thereby avoids or minimizes many deleterious shortcomings of traditional thermal spray methods such as high-temperature oxidation, evaporation, melting, crystallization, residual stresses, gas release. In this process, powder particles are accelerated by the supersonic gas jet at a temperature that is always lower than the melting point of the material, resulting in coating formation from particles in the solid state and hence no melting and solidification process is experienced by the powders like in traditional thermal spray process ( Singh et al., 2012). Moreover, the footprint of the cold spray beam is very narrow typically around 5 mm diameter due to small size of the nozzle (10-15 mm2 ) and spray distance (5-25 mm), yielding a high-density particle beam, results in precise control over the area of deposition over the substrate surface. This process is similar to a micro shot peening and hence the coatings are produced with compressive stresses, rather than tensile stresses, which results in dense and ultra thick (5-50 mm) coatings without adhesion failure. The low temperature formation of coating leads to oxides and other inclusions -free coatings with wrought-like microstructure ( Karthikeyan, 2004). No phase transformation occurs during the cold-spray process since it is a 100 pct solid-state process, implying no particle melting. These features are suitable for a deposition of corrosion resistant coatings.
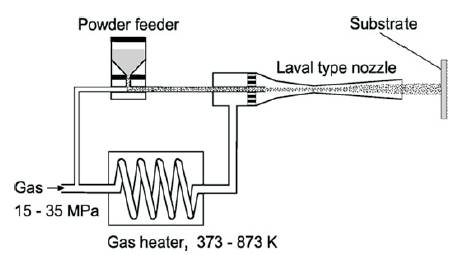
Figure 2. Cold spray coating system
Number of researcher had tested successfully cold spray coatings for different applications, for high temperature protection ( Richer et al., 2010; Zhang et al., 2008), bond coatings for thermal barriers ( Zhang et al., 2008) and for wear and corrosion resistant [ Pardo et al., 2009; Villafuerte & Wenyue, 2007 ). Goyal et al. (2012) study the effects of different parameters of cold spray, on coating thickness and coating density, using Taguchi and analysis of variance (ANOVA). The formation and quality of cold spray coatings depends, mainly on the impact velocity and the initial temperature. Wu et al. (2006) and Schmidt et al. (2006) founded that when the impact velocity of the particle was less than Vcritical (critical deposition velocity) or greater than Verosion (critical erosion velocity), the particles would rebound or erode the substrate surface. They demonstrated schematically how Vcritical and Verosion varied with the initial temperature.
Cold spray is an emerging technology that produces high density metallic coatings with low oxide contents and high thermal conductivity, which makes them ideal for high temperature corrosion resistance. The comparatively low process temperatures, the very short time scales and the use of more or less inert process gases make the cold spray process particularly suitable for applications where it is vital to avoid oxidation and to retain properties of the powder feedstock in the sprayed coatings ( Stoltenhoff et al., 2006). CoNiCrAlY coatings were deposited using the Cold Gas Dynamic Spraying system developed at the University of Ottawa Cold Spray Laboratory by Richer et al. (2008). Coatings were characterized as having low porosity (<2%) and large build-up thickness up to 800 μm. Dense coatings usually provide better corrosion resistance than porous coatings, as the porosity can harm the persistent corrosion resistance of the coating ( Zhao et al., 2004; Zhao et al., 2005 ). Goyal et al. (2010) compare cold spray process feature with other thermal spray process as shown in the Table 1.
Assadi et al. (2003) explain the bonding mechanism in cold spray. They supply a hypothesis for the bonding of particles in cold gas spraying, by making use of numerical modeling of the deformation during particle impact. The results of the modeling provide a basis for understanding the bonding process. Based on these results, the bonding of particles can be attributed to adiabatic shear instabilities which occur at the particle/substrate or particle/particle interfaces at high velocities. The modeling also shows a very non-uniform development of strain and temperature at the interface, suggesting that this bonding is confined to a fraction of interacting surfaces. This result is consistent with relatively low strength of the copper coatings as determined experimentally. The analysis also suggests that density and particle temperature have significant effects on the critical velocity and are thus two of the most influential parameters in cold gas spraying.
Bala et al. (2010) had deposited Ni-20Cr and Ni-50Cr coatings on two boiler steels, namely, SAE 213-T22 and SA 516 steel by cold-spray process. They found that microstructures of the cold-sprayed coatings were found to be dense and packed with interlocked particles generally free of discernible oxide particle inclusions, which is a characteristic of the cold spray process. The porosity for the as-sprayed coatings was found to be in the range of 1.5 to 1.6 pct for both the Ni-20Cr and Ni-50Cr coatings, irrespective of the substrate steel. Further they studied the high-temperature oxidation behavior of the coatings under cyclic thermal exposures at an elevated temperature of 1173.15 K (900°C) to ascertain their high-temperature oxidation behavior. Cold-sprayed Ni-20Cr and Ni-50Cr coatings were useful in imparting high-temperature oxidation resistance to the boiler steel under investigation. The oxide scales of both Ni-20Cr and Ni-50Cr coatings showed no tendency toward spalling/cracking and were found intact, which proves that both coatings possess high adhesion strength. The Ni-50Cr coating was found to be better than the Ni-20Cr coating and reduced the weight gain of the steel by 93 pct. The formation of a protective Cr2O3 phase along with NiCr2O4 in the oxide scale might have imparted a better oxidation resistance to the coating.
The hot corrosion performance of cold-sprayed Ni- 20wt.%Cr coating on SA 516 (Grade 70) boiler steel in a simulated boiler environment of molten salt Na2 SO4 - 60wt.%V2O5 at 900°C under cyclic conditions was reported by Bala et al. (2010). They concluded that cold spray coating of Ni-20Cr alloy powder was found to be useful in developing hot corrosion resistance in SA 516 steel in Na2SO4 -60%V2O5 environment at 900°C. Whereas uncoated steel showed substantial spallation of its oxide scale during hot corrosion studies in the aggressive environment of Na2SO4-60%V2 O5 environment at 900°C. The Ni-20Cr-coated steel after exposure to molten salt has shown the presence of Ni and Cr in its oxide scale, which are reported to be protective oxides. The Ni-20Cr coating was found to be successful in retaining its continuous surface contact with the substrate steel during 50 cycles (each cycle consist of 1 h of heating at 900°C followed by 20 min of cooling at room temperature) Bala et al. (2010) found similar results of hot corrosion performance when Ni- 50Cr powder was deposited on two boiler steels SA-213-T22 and SA 516 (Grade 70) by cold spray process and in Na2SO4-60%V2O5 environment at 900°C. They observed an increase in the resistance towards hot corrosion with the application of coatings. Weight change (mg/cm2) variation as a function of time expressed in number of cycles for Ni–50Cr-coated steels after hot corrosion in Na2SO4 –60%V2O5 at 900°C for 50 cycles is shown in Figure 3. After cold spray coating all the steels showed much lesser overall weight gain as compared to uncoated specimens in the given molten salt environment.
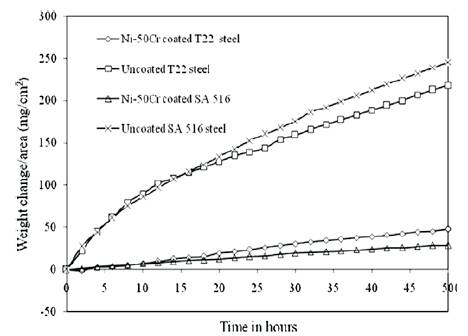
Figure 3. Weight change/area vs. number of cycles plot for the uncoated and cold spray coated Ni–50Cr boiler steels subjected to hot corrosion in Na2SO4 –60% V2O5 environment at 900o C for 50 cycles (Bala et al., 2010).
Richer et al. (2010) compared the microstructure and oxidation behaviour of CoNiCrAlY coatings at 1000°C manufactured by the APS, HVOF and cold gas dynamic spraying deposition techniques. Authors reported that the oxide growth rates is low for both the CGDS and HVOF coatings as a result of low porosity and oxide content. The oxide scale on the CGDS and HVOF coatings after 100 h of oxidation were composed mainly of alumina without the presence of detrimental fast-growing mixed oxides. The presence of Cr2O3 and dispersed NiO was however also observed for the HVOF coating. As expected, the APS coating featured the onset of mixed oxides in the early stages of oxidation. From these results, it appears that potential improvements to the bond coat oxidation behaviour can be achieved using low-temperature processing methods such as CGDS.