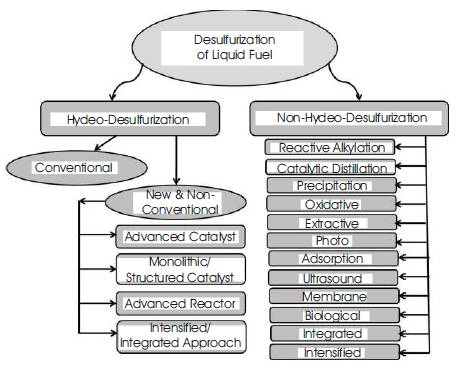
Figure 1. Various Methods for Desulfurization of Liquid Fuels
Extractive desulfurization is an alternative for other desulfurization processes due to its certain merits such as less expensive and green technology. In single stage extractive desulfurization, low level of sulfur (< 50 ppm) in liquid fuel is not achieved. Hence multistage extractive desulfurization is preferred for deep desulfurization (< 50 ppm). In present paper various outlook of multistage extractive desulfurization using ionic liquids has been presented. Feasibility of ionic liquids for multistage extractive desulfurization of liquid fuel with respect to various process parameters has been discussed. Also the extraction mechanism and regeneration along with process consideration has been reviewed.
The major source for increased sulfur oxides into the atmosphere is mainly from the transportation sector. Sulfur ranges from 0.03 wt% to 8 wt% in crude oil [1]. Due to growing strict environmental requirements, sulfur removal from transportation fuels has become a big technical challenge. The new on-road diesel regulation specifies sulfur cap of 50 ppm in many countries [2]. In India, it has been kept as 50 ppm in selected area for BSIV/Euro 4 and 350 ppm nationwide for BSIII/Euro 3 since 2010 http://www.dieselnet.com/standards/in/fuel.php, December 2012).
Extractive desulfurization is an alternative for other desulfurization processes due to its certain merits such as less expensive and green technology. In single stage extractive desulfurization, low level of sulfur (< 50 ppm) in liquid fuel is not achieved. Hence multistage extractive desulfurization is preferred for deep desulfurization (< 50 ppm). In present paper various outlook of multistage extractive desulfurization using ionic liquids has been presented. Feasibility of ionic liquids for multistage extractive desulfurization of liquid fuel with respect to various process parameters has been discussed. Also the extraction mechanism and regeneration along with process consideration has been reviewed.
HDS (hydro-desulfurization) is the conventional and popular technology for the desulfurization of liquid fuel. Presently catalytic processes through hydro- processing are widely used in many refineries. Apart from these processes there are membrane [3], precipitation [4], adsorption [5], oxidative desulfurization [6], photo-oxidation [7], selective extraction [8], reactive alkylation [9], ultrasound [10], and biodesulfurization [11]. Review of various technologies for desulfurization of oil refinery streams can be found in literature [12]. Figure 1 shows typical classification of various desulfurization techniques for liquid fuel.

Figure 1. Various Methods for Desulfurization of Liquid Fuels
Extractive desulfurization is an alternative for HDS and other processes. It can be carried out at or around ambient conditions. Eßer et al. [13] have given various properties for an ideal extracting agent for extractive desulfurization. These properties are: high partition coefficient, easy regeneration or reversible extraction, absolutely insoluble in fuel, almost zero solubility of fuel in extractant, high thermal and chemical stability, non-toxic, and environmentally benevolent [13]. Various solvents like: polyalkylene glycol, polyalkylene glycol ether, pyrrolidones, imidazolidinones, and pyrimidinones have been patented for the extractive desulfurization of the liquid fuels [14]. Now-a-days green chemistry and engineering approach is very popular due to its sustainable applications. Ionic liquids as green solvents have gained the interest in extractive desulfurization of liquid fuels. Bossman et al. [8] used ionic liquids first time for selective extraction of sulfur compounds from diesel fuel. The possible extraction mechanism may be that molecules with highly polarizable π- electron density (such as thiophene) preferably insert into the dynamic molecular structure of the ionic liquids, and the driving force for the molecular insertion is the favorable electronic interaction of polarized aromatic molecules with the charged ion pairs of ionic liquids [15]. The schematic mixer settler process for the extractive desulfurization of liquid fuel using ionic liquid is shown in Figure 2.
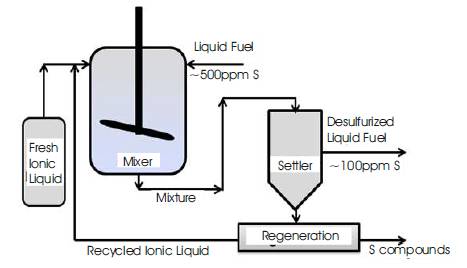
Figure 2. Schematic Process for Extractive Desulfurization of Liquid Fuel Using Ionic Liquids
The ionic liquids (ILs) have been known and studied since last five decades but its application in industrial process has been initiated in last few decades [16] . It has very wide green applications in the field of chemical processes [17]. Exhaustive review on characteristics and application of ILs can be found in the literature [18]. In solvent extraction, solvent loss and toxicity are the major concern due to the organic solvents. Most of the ionic liquids have negligible vapor pressure hence these can be used as a green solvent which are the alternative for conventional organic solvents. As compared to normal liquid, ILs are entirely composed from ions instead of molecules. These ions are bound mainly by ionic bond instead of Van de Waals force. Ionic liquids are non-volatile and have high thermal stability. The physical properties such as density, viscosity, melting point, and hydrophobicity of ionic liquid can be tuned as per our requirement by proper selection of anion and cation [19].
In single stage extraction, liquid fuel (with sulfur ~ 500-1000 ppm) can be taken from storage tank and mixed with ionic liquid. Due to high selective partition of sulfur compounds, sulfur compounds are transferred into ionic liquid. Further, mixture is separated in separator into ionic liquid phase and fuel phase with low sulfur. Ionic liquid can be regenerated using suitable regeneration methods such as: electrolysis, precipitation, dilution with water, heating and vaporization, solvent back extraction etc [20]. The application of ionic liquid for desulfurization of liquid fuel in single stage is discussed in earlier paper [20]. In single stage extraction, the maximum % extraction of sulfur for 1:1 IL to fuel ratio is 70%. In single stage extraction process, the final S-content in liquid fuel does not meet the requirement of low-sulfur (< 50 ppm), hence multistage extraction is required.
It is difficult to reduce the sulfur content to a permitted level (< 50 ppm) in single stage extraction. Therefore, a multiple extraction is desired to investigate the ultimate extractive performance of ILs. In multistage extraction, the desulfurized model oil from the first extraction step is again treated with fresh or regenerated ionic liquid. This may be repeated up to 3-5 times to reach low sulfur level. The typical mixer-settler approach of multistage extractive desulfurization of liquid fuel using ionic liquid is given in Figure 3.
Bösmann et al. [8] studied multistage extraction process in mixer–settler system to reach very low sulfur levels using [BMIM] Cl/AlCl3: 0.35/0.65 for various IL/Fuel ratios and repeated up to four times. They found that a lower mass ratio of model oil to ionic liquid resulted in lower sulfur contents. Below 50 ppm sulfur can be reached for all mass ratios if the number of extraction steps are high enough [8].
The sulfur concentration of the model oil decreases from 498 ppm to 18 ppm after six extraction cycles for multistage desulfurization of the model oil with [BPy]BF4 [21]. Liu et al. [22] carried out five stage extraction for removal of sulfur below 50 ppmw from real diesel oil (438 ppmw sulfur) using [(CH2)4SO3HMIM][ETos]. A multistage extraction was carried out at room temperature with fresh [BMIM]Cl/FeCl3 in continuous working unit for desulfurization of n-octane containing DBT [23] . Very high desulfurization efficiency of 97.9% after four extraction steps, which can meet the requirement of the deep desulfurization was observed.
Three stage extraction for desulfurization of model fuel (160 ppm DBT in n-octane) using various ILs for 3:1 mass ratio of fuel:IL at room temperature for 10 min was studied by Gao et al. [24]. The sulfur content decreased to 89.1, 82.9, 27.3 ppm by [BMIM][PF6], [BMIM][BF4], and [BMIM][FeCl4] respectively from initial 160 ppm. The desulfurization efficiency of [BMIM][FeCl4] was found to be best among these three ionic liquids. The reason is that the Lewis acid-base interaction and Fe3+ can form π-complexation bonding with aromatic sulfur compound [24].
Yu et al. [25] observed remarkably sulfur dropped to 17 ppm after five extraction stages from model fuel (549 ppm sulfur in 85% n-hexane + 15% toluene) by [C2MIM][N(CN)2] for extraction time, 20 min and 1:1 mass ratio at room temperature, 25OC. Almost 97% sulfur removal efficiency was obtained. Chen et al. [26] observed that the sulfur content in gasoline (thiophene in 85% n-hexane + 15% toluene) drops drastically from 558 ppm to 20 ppm after five stages and the sulfur content in diesel (DBT in n-octane) fuel is reduced from 547 ppm to 8 ppm after 4 cycles. The results of multistage extraction for desulfurization of liquid fuel using various ionic liquids are summarized in Table 1.
The linear relationship of sulfur content) vs. the number of extraction steps (Table 1) has been observed which indicates that the extraction can be described by partition coefficient according to Nernst's law. Bösmann et al. [10] confirmed it by re-extraction experiments in which the same trend distribution of sulfur was observed. It was tested by the reextraction of DBT from the loaded ionic liquid [HN(C6H11)Et2][CH3SO3]/[HNBu3][CH3SO3] = 1/1 with in this case S-free n-dodecane. They also confirmed that the extracted DBT remains unreacted hence ILs can be regenerated.
For commercial application of an ILs for the extractive desulfurization of liquid fuels, the regeneration and recycling of ILs is necessary in view of high cost of ILs. The removal of S-compounds from an ILs can be carried out by a variety of ways of which are heating the IL to vaporize the S-compounds at temperatures within the IL stability range [29], back extraction of the S-compounds from the IL with another solvent [13], removing the aromatic S-component by electrolysis [30], or precipitating the S-components by dilution with water [31] . Further, desulfurization by multiple extractions is an effective way to reduce the sulfur content significantly in fuel oils to a negligible amount.
Due to strict environmental regulation, the supply of low sulfur liquid fuel to the transportation sector is worldwide big challenge for petroleum refineries. Extractive desulfurization using ionic liquids is an alternative green process for deep-desulfurization (less than 50 ppm sulfur). Ionic liquids for extractive desulfurization of liquid fuel reported in the literature are moisture-insensitive, thermally stable under the distillation conditions, and readily regenerated for reuse. The sulfur in the liquid fuel can be lowered to desired level by multistage extraction. The removed aromatic S-containing compounds can be recovered by regeneration and ionic liquid can be reused. The higher aromatic π-electron density are the most possible reason for extraction by ionic liquids. The cation and anion structure and size in the ionic liquids are important parameters to decide extraction capacity.
Author gratefully acknowledge for the financial support by the Council of Scientific and Industrial Research (CSIR) grant number (22(0492)/09/EMR-II), Government of India, India.