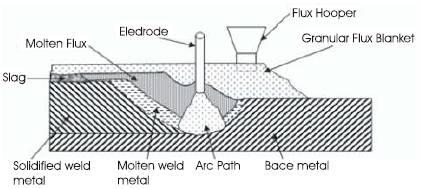
Figure 1. Typical Weld Pool Dynamics of a Submerged Arc Weld
In this paper, the work done by previous investigators for the development of new agglomerated flux used during submerged arc welding process is reviewed, and the gaps are identified. The new fluxes are developed by using CaO, SiO2 and Al2O3 basic elements and with the minor addition of MnO, CaF2, NiO, MgO and Fe-Cr are chosen as variables. The effect of variable flux constituents on weld metal are analyzed in the light of mechanical properties, bead morphology, element transfer analysis and physical properties by using response surface methodology technique. The results indicate that the newly developed flux behavior is at par of commercial fluxes.
Submerged Arc Welding (SAW) is a process in which the arc is concealed by a blanket of granular and fusible flux. Heat for SAW is generated by an arc between a bare, solid-metal (or cored) consumable-wire or strip electrode and the work piece. The arc is maintained in a cavity of molten flux or slag, which refines the weld metal and protects it from atmosphere contamination. Alloy ingredients in the flux may be there to enhance the mechanical properties and crack resistance of the weld deposit.
Figure1 shows the melting and solidification sequence of SAW. A continuous electrode is being fed into the joint by mechanically powered drive rolls. A layer of granular flux, just deep enough to prevent flash trough, is being deposited in front of the arc. Electrical current, which produces the arc, is supplied to the electrode through the contact tube. There can be either Direct Current (DC) with electrode positive (reverse polarity) and with electrode negative (straight polarity), or Alternative Current (AC). After completion of welding and the solidification of weld metal, the unused flux may be screened and reused. The solidified slag may be collected, crushed, resized, and blended back into a new flux. Re-crushed slag and blends of re-crushed slag with unused (virgin) flux are chemically different from the new flux. Blends of re-crushed slag may be classified as a welding flux, but can not be considered the same as the virgin flux.

Figure 1. Typical Weld Pool Dynamics of a Submerged Arc Weld
In this paper, the work of each researcher and their findings (from 1961 to 2011) are described. On the basis of review study, the gaps are identified for the current research.
The American welding society defines welding flux as a material used to dissolve or facilitate removal of oxides and other undesirable substances. Welding slag has no formal definition, but is generally used to describe the fused residue after welding. Submerged arc welding fluxes usually consist of combination of manganese oxide and silica or lime and silica with additions of various other oxides to produce complex oxide fluxes. Lately, fluxes having titania, magnesia, alumina, and fluorspar as the major constituents have also been produced. Submerged arc welding may be produced in one of the three ways: fused, bonded and agglomerated. Jackson [1] gives a detailed description of fluxes and also a list of advantages and disadvantages of each method of production. Apart from the manufacturing method and the active, neutral, or alloy behavior of flux types, another common method used to describe submerged arc fluxes is basicity index, BI. The basicity index is the ratio of strongly bound metallic oxides to weakly bound metallic oxides [2] . The basicity index is an estimate of the oxygen content in the weld metal and is therefore used to predict weld metal properties. Basic fluxes tend to have lower weld metal oxygen content with good weld metal toughness, while acidic fluxes tend to produce higher weld metal oxygen content and coarser microstructure with a lower resistance to cleavage. Fluxes may be considered acidic, basic and neutral [3] . Table1 shows composition ranges of various flux types along with their chemical character and basicity index range.
Lewis et al. [4] developed a new flux and filler wire for submerged arc welding HY-steel used in submarine hulls. The developed flux raises weld metal impact properties by reduction of oxygen, by reduction of inclusions and changes in weld metal microstructure. Pokhodnya and Kostenko [5] studied about fusion of electrode metal and its interaction with the slag during submerged arc welding. They investigated that during submerged arc welding the electrode metal is transferred in the form of droplets but if the current is very high, the metal may be transferred to the weld pool without forming droplets. Bennet & Stanley [6] used CaO-SiO2-CaF2, CaO-SiO2-Al2O3, CaO-SiO2-TiO2 and miscellaneous commercial fluxes for the submerged arc welding of Q.T 35 steel. The study revealed that the flux composition affects the mechanical properties like impact strength and tensile strength very well in the presence of alloying elements or their oxides. Butler and Jackson [7] worked on 75 synthetic fluxes and weld for testing them to ascertain if any compositions were suitable for a submerged arc welding flux. A good welding performance was achieved with the composition of 25% calcium oxide, 30% TiO2, 40% SiO2and 5% CaF2 when used with a filler metal containing 1% manganese. The investigators correlate the welding properties of the various flux compositions with the ternary phase diagram and different characteristics were evaluated, (a) Maximum speed at which the synthetic flux is welded satisfactorily, (b) Appearance and density of synthetic slag, (c) Appearance of fused melt removed from the weld, (d) Electrode melting rate and (e) Flux consumption. Bennet [8] investigated the two aspects: moisture content and rough appearance of weld surface by using basic fluxes. The investigator also compared the slag detachability and weld bead appearance of basic fluxes used in the study with the commercial acid fluxes. P. Colvin [9] in his study stated that the use of basic fluxes in preference to acid fluxes and concluded the use of all mineral non-carbonate basic fluxes with a basicity greater than 2.6 can appreciably extend the scope of the submerged are process by depositing weld metal of improved metallurgical quality without detracting from the physical and economic characteristics of the process. Drayton [10] observed the influence of current, current type and polarity, voltage and flux composition welding parameters on process variables such as metal deposition rates, weld bead profile and flux consumption using single wire for submerged arc welding.
Ivochkin et al. [11] used filler metal in the form of powder and set the submerged arc welding process variables on the basis of calculation and experimental method. Palm [12] investigated that the dissolved oxygen is the deciding factor in determining impact properties of weld metal particularly in the transition temperature and it is controlled by the molten slag and its active components. Potapov and Babin [13] observed an improvement in technological properties with respect to weld bead appearance, reduced gas evolution, improved arc stability and slag detachment etc. by using fused slag as a slag forming base of the agglomerated flux. Sorokin and Sidlin [14] examined the effect of transfer of alloying elements from the electrode into the deposited weld metal. The combined actions of alloying elements and of the oxidizing action of the coating do not affect the degree of adoption of molybdenum and tungsten by the deposited metal. Ferrera and Olson [15] studied the performance of MnO-SiO2-CaO system as a welding Flux. Fluxes, derived from pure components, were tested using the submerged arc welding process. Viscosity, arc stability, and weld-bead morphology were reported and were compared with existing accepted concepts for welding flux behavior. Wittstock [16] gave some guidelines for the selection of electrode, flux and materials used for welding. Eagar [17] identified the source of weld metal oxygen contamination during submerged arc welding. The investigator also elaborated the role of flux basicity index in an indirect measure of the oxygen of the weld metal. Potapov and Kurlanov [18] described a quantitative evaluation of the basicity of welding fluxes. North et al. [19] examined the influence of flux formulation on the oxygen content of submerged arc weld deposit. They used low oxygen potential constituents e.g., CaF2, Al2O3 and CaO for this study and observed their results for fused fluxes and agglomerated fluxes. It is found that oxygen contents were markedly lower using fused fluxes as compared to agglomerated fluxes. Charles and Entrekin [20] observed the influence of flux basicity on weld-metal structure and examined the effects of two fluxes of different basicities when used with the two experimental wires and a columbium-bearing base plate. Schwemmer et al. [21] described the effect of welding flux on weld penetration and established the relationship. Researchers also obtained an expression relating penetration to arc stability, viscosity, and interfacial tensions. Koukabi [22] developed fused fluxes by using CaF2, Al2O3 and CaO and added some more elements like zirconium, vanadium and titanium/boron in flux. The effects of these elements were studied with reference to good mechanical properties, cleanliness and microstructure in submerged arc welds deposits. Chai and Eagar [23] studied two commercial fluxes namely calcium silicate and manganese silicate during submerged arc welding for parametric study of manganese silicon, carbon and oxygen for their recovery. Davis and Bailey [24] described how submerged arc flux composition influences element transferred and analyzed in the light of what has been learned of slag/ metal reactions. Silvinski [25] observed the effects of the components of the CaF2-SiO2-- Al2O3-MgO of flux systems and some oxides on the density, viscosity and surface and inter-phase tensions of slag during interaction with type EP 690 metal. Podgaetskii and Galinich [26] described the structures of molten welding slags. This study established the connection between the chemical composition, the high-temperature physical and chemical properties, and the structure of molten welding slag. Davis and Bailey [27] examined a study of ten separate flux properties at ambient and elevated temperature. The results give a general insight into the relationship between the characteristics and flux behaviors in welding and show specific correlation between coefficient of expansion and slag detachability and between melting point and current capacity. They selected flux composition; bulk density, grain size and flowability for measurement at ambient temperature and their effects were correlated with the welding behaviors. Chai and Eagar [28] studied the stability of metal oxides with reference to oxygen level in the weld metal by producing binary CaF2-metal oxide fluxes. The metal oxides used for this study include SiO2, MnO, MgO, Al2 O3, Na2O, Tio2, K2O and CaO. The study revealed that the stability of metal oxide during welding does not directly depend on their thermodynamic stability because some fluxes even have good thermodynamic stability decomposed into suboxides during welding, such oxides produce higher level of oxygen in the weld metal and reduce weld impact strength. Snyder and Pense [29] observed that the effects of titanium on the mechanical properties and microstructure of submerged arc weld metal in HSLA Si-Al-killed low sulfur steels containing varying amounts of titanium was determined. The outcomes of the study revealed that toughness is strongly dependent on the titanium and manganese levels. Terashima and Tsuboi [30] described the development of submerged arc welding consumables for steel plate of tensile strength above 785 N/mm2. A highly basic agglomerated flux combined with wire containing sufficient Si produces weld metal of high strength and toughness and low diffusible hydrogen content. Kohno et al. [31] developed new fluxes which can consistently micro-alloy weld metal with Ti and B. The developed fluxes improved weld metal toughness for HSLA steels. The developed new fluxes were used during submerged arc welding for the applications of node cans of offshore platforms, LPG tanks and ships. Potapov [32] observed the results by calculating the reactions between slag and metal in automatic submerged arc welding. The equations which have been given for determining the final amounts of silicon, manganese, sulphur, and phosphorus for this process make it possible, with an accuracy sufficient for practical purposes, to calculate the increase in silicon, manganese, sulphur, and phosphorus contents of the weld metal, and consequently to forecast the properties of the weld metal. Davis et al. [33] examined the effects of titanium and boron additions to submerged arc welding fluxes.
Indacochea and Olson [34] established the relationship of weld-metal microstructure and penetration to weld-metal oxygen content. Mitra and Eager [35] studied the transfer of Cr, Si, Mn, P, S, C, Ni and Mo between the slag and the weld pool for submerged arc weld for low alloy and stainless steels with calcium silicate and manganese silicate fluxes. Lau et al. [36] studied the sources of oxygen and nitrogen contamination during submerged arc welding of different CaO-Al2 O3 based fluxes of varying basicity index (B.I.). Lau et al. [37] studied about gas/ metal/ slag reaction in submerged are welding using CaO-Al2O3 based fluxes, and they work on the interaction of Mn, Al and O at the different stages of the welding operation. According to them, at the electrode tip and at the arc column, changes in oxygen, aluminum and manganese were dominated by flux decomposition, while at the metal stage, slag- metal reaction occurred. Tandon et al. [38] observed the effect of flux characteristics on HAZ during submerged arc welding. In this study fluxes, based on CaO-TiO2 system with minor additions of Al2O3, MgO and CaF2 of different basicities have been designed and manufactured by fusion method. The physical properties like melting point, melting range and volumetric specific heat have been determined. Burck et al. [39] observed the effect of welding flux additions on 4340 steel weld metal composition, they stated that the effects of CaF2, CaO and FeO addition on weld metal chemistry were evaluated for the manganese silicate flux system. Comparisons were made between AISI 4340 steel and low carbon steel welds to understand the weld metal chemistry. The results show the elemental transfer from the slag to weld metal and vice versa and cannot be consistently explained using thermodynamic data. Mitra and Eagar [40-42] studied a critical review of thermodynamic theories of slag-metal reactions. The authors established a quantitative relationship between the compositions of welding consumables used, the welding parameters employed, and the resulting weld metal chemistry. In part II, an entirely new theory was presented to explain the changes in weld chemistry, and a kinetic model was formulated to predict weld metal composition. The part III, verified the theory through several different experiments. Gupta and Arora [43] examined the weld bead geometry and heat affected zone which are generally dependent on the welding parameters and may also be influenced by the chemical composition of flux. The authors also investigated that the depth of penetration, weld bead width and weld reinforcement depend upon welding parameters and flux basicity. Pandey et al. [44] observed the effect of submerged arc welding parameters and fluxes on element transfer behavior and weld metal chemistry. Paniagua et al. [45] studied on chemical and structural characterization of fluxes for submerged arc welding. Three flux formulations were preferred using mineral oxides for agglomerating and sintering process. A commercial agglomerated and sintered flux was used for comparison. The four flux were analyzed chemically by atomic absorption and X-ray diffraction to determinate the quality and type of oxide formed, as well as the change in oxidation number after the sintering processes at 9500C of the initial compound. Differential thermal analysis was carried out from 1000 to 13500C in order to determine the temperature for phase transformations and melting of the different compound formed in the sintering process. Paniagua [46] observed the effect of flux composition for the microstructure and tensile property of SAW in AISI 1025 steel. Kanjilal et al. [47] developed model by rotatable design technique to study the combined effects of flux and welding parameters on chemical composition and mechanical properties of submerged arc weld metal. Kanjilal et al. [48] studied the prediction of element transfer across the molten pool in submerged arc welding by developing quadratic models in terms of flux ingredients with the application of statistical experiments for mixture design. Adeyeye et al. [49] used mixture experiments optimization technique to design flux for submerged arc welding. Abhay Sharma et al. [50] developed a mathematical modeling of flux consumption during twin-wire submerged arc welding. As the process signifies, during welding a certain amount of flux is consumed and converted into slag and therefore the flux consumption remains a function of process parameters and directly influences the productivity of the process. Sahni et al. [51] used slag waste as a flux in submerged arc welding. The result of this flux does not adversely affect the chemistry of weld metal. Bang et al. [52] studied the effects of wire/flux combination on the chemical composition, tensile strength, and impact toughness of the weld metal. P Kumar et al. [53] observed the effect of flux, welding current, arc voltage, and travel speed on changes in micro-hardness and microstructure of the Heat-Affected Zone (HAZ) and to optimize the process. Vinod Kumar [54] developed mathematical model for submerged arc welding using developed fluxes. He also used response surface methodology to predict critical dimensions of the weld bead geometry and shape relationship.
After literature review the following gaps are identified.
A number of investigators designed and developed different agglomerated fluxes with minor additions of alloying constituents during submerged arc welding, but every time they keep in mind the objective of welding industries and try to develop fluxes that are not only economically viable but have good techno-mechanical properties, less reinforcement, more weld bead penetration at the weld joint. On the same trend, the present investigators are using CaO- -SiO2 - Al2O3 based flux systems for the study as these are the most widely used fluxes at the commercial level. The ranges of these constituents are designed on the basis of binary and ternary phase diagrams for different oxide and fluoride systems as given in Table 2. After ascertaining their ranges, fluxes are prepared by agglomeration method. The fixed process parameters of submerged arc welding are selected for this study. The Response Surface Methodology (RSM) technique is used and total 32 experiments [55, 56] were conducted on low carbon steel plates of 300X300 X20 mm size (V-groove at 450) by using developed agglomerated fluxes. All the weld joints made are given in Figure 2, by submerged arc welding machine as shown in Figure 3, with a flux depth and electrode stick-out at 25 mm, 500 A and 36V DC electrode positive at 280 mm/min travel speed, with a wire diameter of 3.15 mm, giving a heat input of about 3.87 kJ/mm. Variables MnO, CaF2, NiO, MgO and Fe-Cr and their levels are given in Table 3.

Table 2. Flux Constituents and their Ranges

Figure 2. Weld Joints on Low Carbon Steel Plate
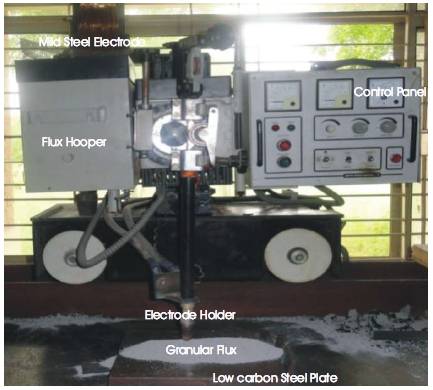
Figure 3. Submerged Arc Welding Set-up
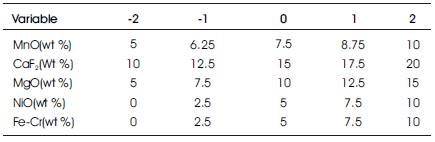
Table 3. Flux Constituents Used in the Experiment and their Levels
The mechanical properties of the weld metal, element transfer analysis, bead morphology and the physical properties of agglomerated fluxes are determined by using newly developed agglomerated fluxes during submerged arc welding process. The result shows that the techno-mechanical properties of weld joint by using developed agglomerated fluxes during process are superior to the base metal and their ranges are given in Table 4. During the element transfer study, the carbon equivalent to all the welded joints by using developed agglomerated fluxes are less than i.e. 0.45, the value of high susceptibility of cold cracking which indicates that these developed agglomerated fluxes are less prone to cold cracking at the welded joints. The physical properties i.e. Grain size, bulk density, viscosity and flowability are at par of commercial flux.
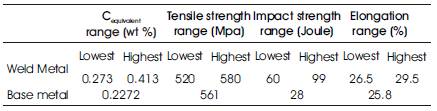
Table 4. Mechanical Properties of Weld Metal Using Developed Agglomerated Fluxes