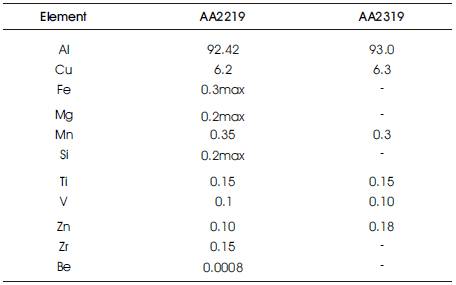
Table 1. Chemical Composition of Base Metal (AA2219) and Filler Wire (AA2319)
Characterization of AA 2219 weldments in T6 condition has been presented in this paper. Automatic TIG welding process has been employed to weld 6.5mm thick 2219-T87 plates. 2319 filler wire has been considered for welding. Tensile and microhardness values have been studied and results confirmed by metallography. 2219 wrought aluminium alloy derives its high strength from the precipitation of CuAl2. T6 has been given as the post weld heat treatment condition to regain the strength lost during the welding.
Aluminium alloy is dominated by aerospace industry. Even though aluminium alloys are significantly more expensive than ferrous alloys there is a continuous requirement to reduce vehicle weight and increase fuel efficiency. Wrought Al-Cu alloys were developed in 1920s and have been used for manufacturing of aircraft structures. 2219 alloy has been extensively utilized in aerospace applications because of their good tensile and creep strength along with better ductility, weldability and cryogenic properties. This alloy possesses best elevated temperature strength (250 – 300oC) and corrosion resistance than all other aluminium alloys [6]. Applications of 2219 alloy are structural components such as rocket shells, cryogenic tanks and engine casings. 2219 wrought aluminium alloy derives its high strength from the precipitation of CuAl2 along slip planes and grain boundaries. With increase in percentage of Cu, above 4.5% in 2219 alloy composition the hot cracking sensitivity of weldment decreases. Hot cracking sensitivity in these Al-Cu alloys increases as copper is added up to 3% and decreases when the copper is above 4.5% [3].
TIG welding is cheapest and easy to use process which can provide quality welds for aluminium alloys [4]. Because of its limitted heat input TIG welding is more suitable for joining thin sections. It can also be used for joining for thin sheets of butt joint without the addition of filler metal. As this is very clean welding process, it can be used to weld reactive metals like Titanium, Zirconium, Magnesium and Aluminium. Solidification cracking and strength loss in weld metal and HAZ are the important subjects to be considered during TIG welding. Hydrogen cracking is common with carbon steels but hydrogen cracking will not occur with aluminum [5]. Hot cracking or solidification cracking is a primary cause for aluminum cracks. Preheating, interpass temperature, weld heat, weld speeds all these are influencing the weld structure and weld strength. The loss of strength after welding due to the HAZ and the weld zone can be improved by post weld heat treatment (PWHT) [2].
The starting material considered for TIG welding study is T87 AA 2219 plates. The dimensions of the plate used for TIG welding process is 1000x300x6.5mm. Electrode considered is non consumable Zirconated tungsten electrode. The filler wire used is AA2319 with diameter of 1.6mm. Helium and Argon have been used as shielding gases. The chemical composition, of base metal and filler wire are given at Table 1.

Table 1. Chemical Composition of Base Metal (AA2219) and Filler Wire (AA2319)
Welding process used is TIG to weld two 6.5mm thick 2219 (T87) plates to form a joint. Melting temperature range of 2319 filler wire is 543 to 643oC. Welding has been carried out across the rolling direction of the plate. Welding of 2219 plate has been carried out as per the weld parameters given at Table 2. Weld joint design used is butt joint as shown in Figure 1.
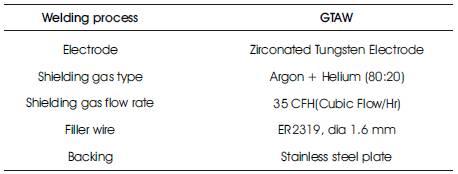
Table 2. Weld Parameters used for TIG Welding
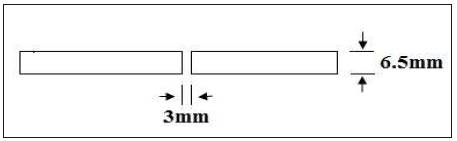
Figure 1. Butt Joint Weld Design for TIG Welding
The Zirconated tungsten electrode and 2219 plates are acid pickled, water and acetone washed and finally wire brushed just before welding. Three passes were used to complete the weld. Aluminum alloy weldments are susceptible to porosity. Hydrogen dissolved in the liquid weld metal will try to escape as the aluminum solidifies and the trapped hydrogen will result in weld porosity. The main cause of porosity in aluminum welds is the absorption of hydrogen in the weld pool which forms gas pores in the solidifying weld metal. The most common sources of hydrogen are hydrocarbons and moisture from contaminants on the aluminum base metal and on the filler wire surface. Utilisation of shielding gas will reduce the porosity formation.
Al-Cu alloys acquire their required mechanical strength through thermal treatment, solution treatment and aging. Post weld heat treatment after welding helps in regain the strength lost during the welding. Through the solution treatment care must be taken that all the alloying elements gone into solid solution and result in supersaturated solid solution. The aging treatment has to be long enough to permit a controlled amount of precipitation of the aluminum alloying elements. The PWHT given for 2219 weldments is presented below in Figure 2.
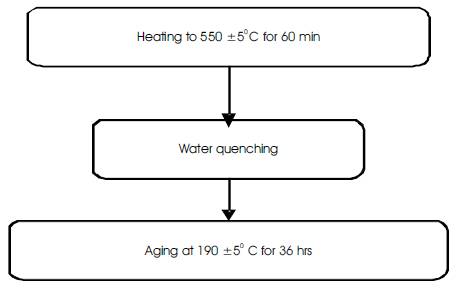
Figure 2. Flowchart of Heat Treatment (T6) Process
The effectiveness of the precipitation hardening can be reduced significantly by heating during welding in the area called the heat-affected zone (HAZ), where the peak temperatures are too low to cause melting but high enough to cause the microstructure and properties of the materials to change significantly. A common method of determining the width and extent of the HAZ is by measuring the hardness across this zone.
Five sequential structures can be identified during the aging of Al–Cu alloys.
Supersaturated solid solution GP → θ'’ → θ’→ θ (Al2Cu)
Where θ (Al2Cu) is the equilibrium phase with a body-centered-tetragonal (bct) structure. The GP zones (Guinier–Preston), the θ'' and θ' are metastable phases. Strengthening is due to the formation of localized concentrations of copper atoms forming Guinier-Preston zones that are structurally coherent with the aluminum matrix. At longer aging times and higher aging temperatures the number of Guinier-Preston zones increase and lead to increased strength; the maximum strength is regarded as the peakaged condition. At still higher temperatures or longer times the Guinier-Preston zones are replaced by the noncoherent, metastable precipitates (θ'' and θ'). The decrease in the amount of Guinier-Preston zones formed and the loss of particle coherency result in a subsequent reduction of strength, termed the overaged condition [1].
The hardness profile for 2219-T87 exhibits dissolution of precipitates. The strengthening particles for these alloys are Guinier-Preston zones, which are metastable precipitates. At positions close to the fusion zone, higher temperatures are experienced and greater dissolution of strengthening phases occurs. This results in a continual decrease in strength in the HAZ of aluminum copper alloys.
Since the chemical composition and local cooling rate are factors that determine the solidification paths and which phases will form [7], it is important that the microstructure of 2219 weldments be fully defined. Specimens cut from the welds before and after heat treatment and from HAZ before heat treatment was prepared for metallographic examination. Etching the polished specimens with freshly prepared Keller's reagent revealed the microstructure.
It is clear from the base metal microstructure, Figure 3 that equiaxed grains of Al solid solution is present due to the cold working condition, T87, which results in good mechanical properties. The heat affected zone (HAZ) is characterized by a coarsened grain structure compared to that of the base metal. This is due to the high heat input, lower cooling rate. The grain size in the HAZ increases as the fusion boundary is approached. The closer to the fusion boundary, the higher the peak temperature becomes and the longer the material stays at high temperatures. Since grain growth increases with increasing annealing temperature and time as shown in Figure 4 a.

Figure 3. Optical Micrograph of AA2319 base Metal, showing Equiaxed Grains of Al Solid Solution
When welding with a filler metal, the weld metal composition is different from the base metal composition, and the weld metal micro structure can differ from the base metal structure. In fusion welding the existing base-metal grains at the fusion line act as the substrate for nucleation and new grains are being nucleated at the fusion boundary. Figure 4 b shows solid dendrites and the interdendritic liquid because of the eutectic type phase diagram is common among aluminum alloys, which results in poor mechanical properties of the weldmetal. The improvement of Mechanical properties has been achieved by the PWHT (T6). In Figure 4 c and d It is clear that very fine precipitates formed a as a result of PWHT at higher magnification, It is important that the weldzone microstructure has shown considerable change and it is almost equal to the base metal structure, by eliminating the dendritic structure.

Figure 4. Optical Micrographs of AA2219 Weldments
The 2219 aluminum plate, T87 condition may contain only one θ' metastable phase before welding. Since the composition of alloy 2219 is beyond the maximum solid solubility, large θ particles are still present after heat treating, but the matrix is still θ containing fine θ' precipitate. The volume fraction of θ' decreases from the base metal to the fusion zone, because of the reversion of θ' during welding. The reversion of θ' is accompanied by coarsening; that is, a few larger θ' particles grow at the expense of many small ones. The presence of such coarse precipitates suggests overaging. Weld zone is heated to an even higher temperature and θ' fully reverted. The cooling rate here is too high for reprecipitation of θ' to occur during cooling to room temperature. The θ' reversion causes the hardness to decrease in the HAZ, which is evident in the as-welded condition [7]. During post weld heat treatment, the GP zones form in the solutionized area near as weld position, causing its hardness to increase significantly because of because of θ'' and θ' precipitation and has been shown in table.
Microhardness survey has been done using Vickers microhardness tester. Values taken across weld, HAZ and interface. Microhardness values are presented in the Table 3. Load considered 200g. Microhardness survey was taken from weld metal towards the base metal. In the non-heat treated specimen the hardness increases from the weld metal towards the base metal but in the heat treated specimen the hardness of the weld metal was almost equal to the base metal shown in Figure 5. This is because during precipitation hardening and aging the weld metal will try to attain the properties of the parent metal.
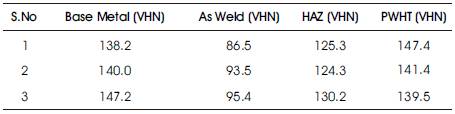
Table 3. Microhardness Values for AA2219 Weldments
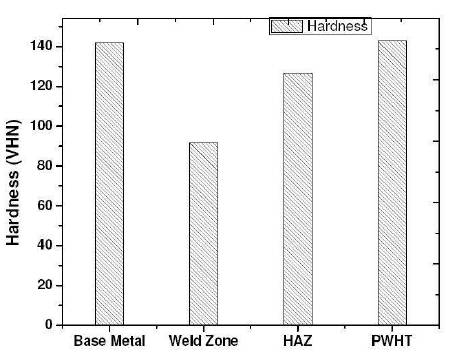
Figure 5. Barcharts showing the Variation of Hardness from base Metal to PWHT
For mechanical-strength standpoint it is essential that, during alloy fabrication, copper should be fully dissolved into the aluminium. It is the solid-solution, or homogenising, treatment that dissolves the copper, and is performed in the temperature range 530-540oC. Cooling from this temperature must be speedy to prevent the formation of the intermetallic compound CuAl2 at the grain boundaries. If excessive numbers of CuAl2 intermetallics do form at grain boundaries they are surrounded by adjoining volumes of alloy depleted in copper, and this will facilitate lower mechanical strength.
Tensile testing of 2219 weldments has been conducted in accordance to ASTM E8 specification. Values of ultimate tensile strength, yield strength and percentage elongation are presented at Table 4. Tensile properties have been tested in as weld condition and in PWHT condition also. Fracture occurred at weld location both for as weld and PWHT samples.
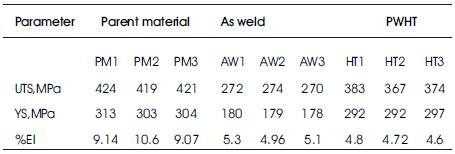
Table 4. Tensile Properties of Parent Metal, as Weld and PWHT of AA2219
The increase in UTS and yield strength after PWHT is due to the formation of very fine precipitates of CuAl2 which results in improved mechanical properties by hindering of dislocation movements are shown in Figure 6.
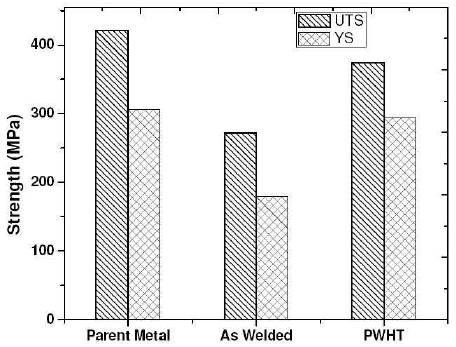
Figure 6. Barcharts showing the Variation of Tensile Properties
The following conclusions have been drawn from the present study AA 2219-T87 plates of 6.5mm thick have been successfully welded by TIG welding using 2319 filler wire. Post weld heat treatment, T6 has been given to the weld 2219 plate to retain its strength lost during welding. This treatment shows a substantially improvement in strength, microhardness of the weld zone. This is attributed to formation of CuAl2 precipitates during aging.
The authors reward their thanks to BrahMos Aerospace Pvt. Ltd. Thiruvananthapuram, for providing support and permission for carrying out this R&D work.