Figure 1. Typical classification of desulfurization methods.
Now-a-days it is mandatory to provide the low sulfur liquid fuel due to environmental regulation by various countries for the transportation sector which is the big challenge for worldwide refineries. In this present paper, extractive desulfurization using ionic liquids has been discussed. It is an alternative process for the high energy intensive HDS technology for deep-desulfurization (less than 50 ppm sulfur). Various aspects of the process has been discussed such as extraction time, fuel to ionic liquid ratio, extraction mechanism, and regeneration along with process consideration.
Due to the industrial revolution, emissions of sulfur have increased. These emissions and those from the transportation sector are the major source for increased sulphur oxides into the atmosphere. The sulfur content of crude oil from different sources ranges from 0.03 wt% to values as high as 8 wt.%. Government regulations in many countries call for more environmentally friendly transportation fuels with lower contents of sulfur and aromatics. Over the last few decades, environmental regulations focus attention on reduction of emissions from the transport sector with the purpose of improving air quality and welfare [1] . Babich and Moulijn [2] discussed about various external and internal factors which influences modern refineries to get the fuel of high quality at reasonable price.
Sulfur removal from transportation fuels has become an increasing technical challenge as oil refineries face growing environmental pressures and strict regulatory requirements. In many countries, the new on-road diesel regulation specifies sulfur cap of 15 ppm. The European Union has stringent fuel quality rules that require maximum diesel sulfur content of 50 ppm in 2005 (350 ppm in 2000), and maximum petrol (gasoline) sulfur content of 50 ppm in 2005 (150 ppm in 2000) [3] .
Refinery industry utilizes widely catalytic processes for desulfurization of fuels through hydro- processing. While the performance of conventional hydroprocessing catalysts have been highly effective for the reduction of sulfur levels, further removal of residual sulfur from the processed fuels is seen to largely increase the cost of hydroprocessing [4] . The processes are highly energy intensive and consume large amount of hydrogen, so it is difficult to generalize. Babich and Moulijn [2] presented an exhaustive review on various technologies for desulfurization of oil refinery streams. The typical classification is given in Figure 1. The removal of sulfur strongly depends on the molecular structure of sulfur compounds. In the literature, apart from HDS, reactive alkylation [5] ), precipitation [6] ., oxidative desulfurization [7] ., photo-oxidation [8] ., adsorption [9] ., selective extraction [10] ., membrane [11] ., ultrasound [12] ., and biodesulfurization [13] . have been studied for desulfurization.
For the extractive desulfurization of the liquid fuels, some molecular solvents such as polyalkylene glycol, polyalkylene glycol ether, pyrrolidones, imidazolidinones, and pyrimidinones have been patented [14] . Extractive desulfurization can be carried out at or around ambient temperature and pressure and without the need for hydrogen [15] . Eßer et al. [15] have discussed the various properties for an ideal extracting agent for desulfurization.
Recently, the extraction of the sulfur compounds by ionic liquids has gained the research. The use of ionic liquids for the selective extraction of sulfur compounds from diesel fuel was described by Bossman et al. [10] for the first time. For solvent extraction method, volatility of most solvents may lead to solvent loss and toxicity concern. This problem is solved by ionic liquid as a new type of solvent with negligible vapor pressure. In contrast to normal liquid, ionic liquid is composed entirely from ions instead of molecules, which are bound mainly by ionic bond instead of van de Waals force. It strongly resembles ionic melt that may be produced by heating metallic salts. Ionic liquids are non-volatile, have high thermal stability and have recently gain interest in new solvent applications. The physical properties such as density, viscosity, melting point, and hydrophobicity of ionic liquid can be tuned by careful choice of anion and cation [16] .
The Ionic Liquids (ILs) has been known since last 40 years and its application in industrial process initiated in last 15 years [17] . Ionic liquids can be used for possible green chemical processes, such as liquid/liquid extractions, gas separations, electro- chemistry and catalysis etc. [18] . Feng et al. [19] have given exhaustive review on characteristics and application of ILs. The scope of utilization of properties of ILs for various novel applications are described in Figure 2 [19] . A few results of extractive desulfurization of liquid fuel using various ionic liquids are summarized in Table 1.
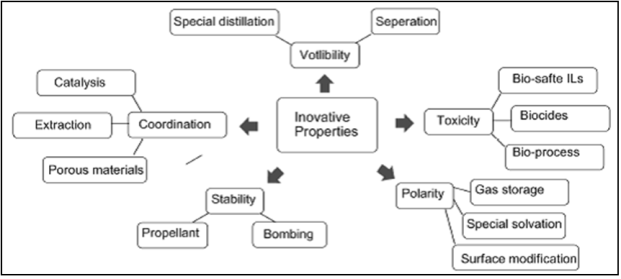
Figure 2. Discovery of innovative properties of IL inducing novel applications
The typical extractive desulfurization of liquid fuels using ionic liquids is discussed by Babich and Moulijn [2] which is based on that sulfur compounds are more soluble in appropriate solvent. The generalized conceptual process for extractive desulfurization of liquid fuel using ionic liquids is shown in Figure 3. Liquid fuel containing sulfur compounds are transferred from the fuel storage tank to mixing tank where it mixed with the ionic liquid. Due to higher selective solubility of sulfur compound, the organic sulfur compounds from the liquid fuel transferred into the ionic liquid. Consequently, the ionic liquid – fuel mixture is fed into a separator in which ionic liquid loaded with sulfur compound is separated from fuel [4] .
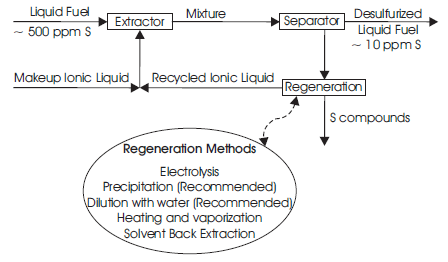
Figure 3. The process for extractive desulfurization of liquid fuel using ionic liquids
Wang and Li [4] give the possible extraction mechanism for the use of ionic liquids in desulfurization. It may be that molecules with highly polarizable π- electron density (such as thiophene) preferably insert into the dynamic molecular structure of the ionic liquids, and the driving force for the molecular insertion is the favorable electronic interaction of polarized aromatic molecules with the charged ion pairs of ionic liquids. Therefore, the thiophene sulfides were retained in the ionic liquids, and then the diesel will become lower-sulfur.
The desulfurized fuel stream can be used for blending with final product or as a further process. The organosulfur compounds can be separated by suitable method [4] and hence ionic liquid can be recycled. Low temperature and low pressure of operation makes this process most attractive for desulfurization. Also the mixing tank can even be operated at ambient conditions. In this process, the chemical structure of the fuel components will not change. It is proven that, with regard to those sulfur compounds that are very difficult to remove by common hydrodesulfurization techniques can be removed by extraction of sulfur using ionic liquids. Traces of such sulfur compounds could easily be removed. The application of very mild process conditions (low pressure and temperature) is an additional advantage of this approach in comparison to traditional HDS [10] .
In order to investigate the time needed to reach the extraction equilibrium various experiments have been reported. Zhang et al. [28] verified that 30 min is more than sufficient to establish the equilibrium and the equilibrium reached after 10 min of contact between the model fuel phase and the ionic liquid phase. Similar results were obtained by other researchers, where the required extraction time ranged from 10 min to 25 min. A short equilibrium time is very necessary to generate high production yield or meet the requirement of small-volume equipment in industrial applications [26] . The short extraction time can be attributed to the low viscosity, which facilitates better dispersion of IL in oils and accelerates the rate of mass transfer.
Considering the high cost of ILs, it is preferable to use low quantity of ILs in fuel desulfurization. Hence, it is necessary to study the effect of Fuel to IL ratio on desulfurization. The extraction yield decreased linearly with an increased in the mass ratio. It implies that an extremely low sulfur level might be attained by multiple extractions or changes in the mass ratio. Although the lower mass ratio of model diesel to ILs, higher the cost of ILs, and the problem may be resolved by the ILs property that is recycling [27].
Eßer et al. [15] studied the effect of sulfur compounds on desulfurization of liquid fuel using ILs. They used dodecanethiol, tetrahydrothiophene, thiophene, BT, DBT, 4-MDBT, 4,6-DMDBT as sulfur source. Almost same results observed for simultaneous extraction [15].
For the technical application of an ionic liquid extraction, the regeneration and subsequently recycling of ionic liquid is of vital importance. For the commercial application of an ILs for the extractive desulfurization of liquid fuels, the regeneration and subsequent recycling of ILs is essential by considering the high cost of ILs. The removal of S-compounds from an ILs can be conducted by a variety of techniques (Figure 3) [29] . These are from heating the IL to vaporize the S-compounds at temperatures within the IL stability range [28] , back extraction of the S-compounds from the IL with another solvent [15] , removing the aromatic S-component by electrolysis [30] , or precipitating the S-components by dilution with water [31] .
Nie et al. [29] suggested S-compounds in an IL can be conveniently separated out by water dilution, and the IL can be reclaimed after vaporization of water at higher temperature. Regeneration of IL via water dilution although technically possible does not seem economically feasible considering the intense energy expenses for the subsequent water removal by evaporation. Therefore, other efficient techniques for separating water.
To provide the low sulfur liquid fuel due to environmental regulation by various countries for the transportation sector is the big challenge for worldwide refineries. Extractive desulfurization using ionic liquids is an alternative process for the high energy intensive HDS technology for deep-desulfurization (less than 50 ppm sulfur). Extractive desulfurization using ionic liquids is promising technology as almost all ionic liquids reported in the literature are moisture-insensitive, thermally stable under the distillation conditions, and readily regenerated for reuse. The sulfur in the liquid fuel can be lowered to desired level by multistage extraction. The removed aromatic S-containing compounds can be quantitatively recovered during the regeneration and ionic liquid can be reused. The results suggest that compounds with higher aromatic π-electron density are the most possible reason that those can be extracted by ionic liquids. The cation and anion structure and size in the ionic liquids are important parameters affecting the extraction capacity for aromatic sulfur compounds.
Author gratefully acknowledge for the financial support by the Council of Scientific and Industrial Research (CSIR) grant number (22(0492)/09/EMR-II), Government of India, India (Principal Investigator: Dr. Kailas L. Wasewar).