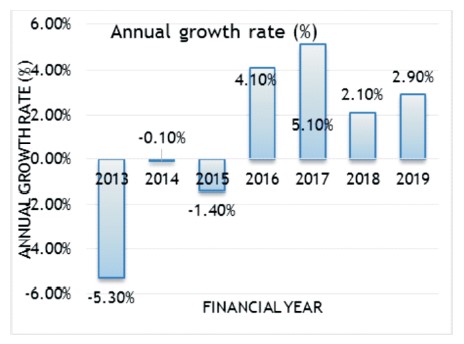
Figure 1. Annual Growth Rate in Mining (Stipp, 2020)
Mineral exploitation and mining are expanding with increasing industrialization, and as exploitation increases, so will their enormous environmental impact. The biological technique was found to be a suitable alternative for treating mine wastes and recovering toxic heavy metals. Acid Mine Drainage (AMD) or Acid Rock Drainage (ARD) is the most well-known mining waste laden with heavy metals that remains untreated. Microorganisms help in detoxification and thereby facilitate the extraction of pollutants from mine waste. Sulfate Reducing Bacteria (SRB), among all known microorganisms, play an important role in mine waste treatment by neutralizing acidity and reviving alkalinity. The use of microorganisms in treating overburden dumps helps reduce the amount of waste, augment natural resources via metal recovery, and maintain a healthy environment. Such a technique picks up momentum due to its low cost, easy availability of ingredients, and eco-friendly nature. Such a treatment system may or may not be capable of removing toxicity. Therefore, it is advisable to use the same along with other techniques depending upon site conditions, the nature of the deposit, and the availability of essential requisites. This paper attempted to highlight potential thrust areas requiring this technique as well as limiting factors.
Mining and environmental degradation move side by side. The exploitation of natural resources is essential for the sustenance of civilization. At the end of the fiscal year 2019, 2.9% of growth (Figure 1) was observed in domestic mining production (Dominguez-Benetton et al., 2018; Bryant, 1987). Mining and mineral processing industries generate huge volumes of waste that are disposed of in tailings dams. Mining exposes radioactive elements that produce fibrous minerals and metallic dust. Dust generation happens due to drilling, blasting, excavation, loading, and transportation of minerals. Tailings slurries may contain toxic and radioactive elements that leak into the strata during separation. Underground mining involves large-scale movements of waste rock and vegetation, directly or indirectly affecting the area's flora and fauna (Dwivedi & Soni, 2011). Furthermore, the effects of mining on the surrounding surface and groundwater are concerning. A high concentration of arsenic, sulphuric acid, and mercury produced during underground mining poses a severe threat to the surrounding surface and groundwater.

Figure 1. Annual Growth Rate in Mining (Stipp, 2020)
Sustainable mining refers to the reduction of the negative environmental, social, and governance impacts of mining operations. Sustainable miners practice responsible stewardship of the natural environment, meeting society's requirements for resources today while ensuring the needs of future generations can also be met. Sustainable mining is when essential resources are extracted while improving social, economic, and environmental outcomes. These undesirable chemicals discharged over vast areas may percolate downward and contaminate groundwater. Ground and surface water contamination will further increase with the disposal of the water used in the mine for drainage, machine cooling, aqueous extraction, and ancillary activities. Oxidation of iron sulphide plays a significant role in this. Hardness, most commonly the sulphide ones, results in the generation of acid mine drainage. However, coal cannot be targeted directly, but pyrite's presence in coal contributes to AMD generation (Park et al., 2019). Improper waste management and left-out ore have significant footprints on the environment. Mining causes enormous loss in all domains like air, water, soil, etc. Underground mining yields vast amounts of waste that are brought to the surface. The waste rock may be toxic or become toxic when it meets water and air. It causes subsidence as mines collapse, and the land above it starts to sink. This causes severe damage to buildings. Mining also causes significant damage to groundwater by lowering the water table, changing groundwater flow, etc.
Mine dust irritates tissues, resulting in anthracosis, also known as "Black Lung," in coal miners (Agate, 1996; Mishra & Sahu, 2013). Adding to the list, noise pollution may also cause health disorders like cardiovascular disorders, hypertension, hearing loss, sleep disorders, and other disturbing effects. Along with mining, construction industries are equally responsible for acid mine drainage. It may also happen in some natural environments. One such example is ARD (Acid Rock Drainage). When acidgenerating rock is crushed and exposed to oxygen and the environment, acid generation starts. This process may continue for thousands of years until the acid-generating stuff (sulphides) gets completely exhausted. The process itself is difficult to stop. The increased acidity due to acid mine drainage has adverse effects. These effects can be categorized depending on the severity of the pH change. Rivers near abandoned mine sites become inhospitable to aquatic life in many cases. Mine discharge laden with these chemicals causes damage to surrounding vegetation. Along with the harmful effects of increased acidity, one additional problem was created due to acid and neutralizing rock (Mishra & Sahu, 2013). Mining waste disposal is necessary, but at the same time, it may cause long-term damage to groundwater, the environment, etc. A minimal and thin coal seam, upon mining, causes large-scale damage to the environment, especially the aquatic environment (Park et al., 2019). Environmental damage mainly depends on the type of mining technique, ore type, blast pattern design, etc.
The biomining technique serves as an alternative to conventional mining, processing, and water treatment. The need for more of the same is the result of several factors, including the depletion of natural resources and increasing environmental degradation, particularly from sulphide ore smelting. The bioremediation process highlights the use of micro-organisms to degrade environmental contaminants. It is one of the most widely accepted techniques among these new technologies. Multiple and versatile applications of bioremediation include the clean-up of surface water, soils, water bodies like lagoons, mill sludges, and various process wastes. Naturally occurring consortiums, mostly dominated by bacteria, can degrade a wide spectrum of environmental pollutants. Such consortiums are responsible for the cleanup of massive oil spills (Lloyd, 2002).
Microorganisms are microscopic by nature. They may be single, multiple, or occur in clusters. They may be beneficial or cause serious harm. They are available in good numbers. Microorganisms grow on their own and form colonies. Their metabolism results in an increase in cell size. Both physical and chemical conditions should be favorable for significant growth. For successful growth, requirements include an abundant supply of water, mineral elements, other growth factors, and gases, particularly oxygen. Proteins, fats, carbohydrates, or lipids are present as chemical substances. All these contain carbon in some form. The growth rate of microorganisms is influenced by temperature, osmotic pressure, acidity or alkalinity, and oxygen concentration as well. These factors can otherwise be termed environmental factors. It can be summarized that physical, chemical, and environmental conditions favor bacterial growth.
Based on temperature preferences, microorganisms are classified into various groups. The best growth is observed at optimum growth temperature. In general, cooler temperatures slow down activity and vice versa. The extent of acidity or alkalinity also determines the enhancement rate. In general, an acidic environment promotes microbe growth, whereas an alkaline environment inhibits microorganism growth. In a nutshell, the environment should not be too acidic nor too basic in nature. Species like Azotobacter are found in the soil and obtain nitrogen directly from the atmosphere, mixture of nitrogen and phosphorus are required for nucleic acid (Kuyucak & St-Germain, 1994). Acting as an electron acceptor, aerobic bacteria use oxygen during the process of cellular respiration. For aerobic organisms, oxygen is required for their energy-yielding properties. Other than the essential ones, microbial growth requires trace elements such as iron, copper, and zinc. These elements are often used for the synthesis of enzymes. At times, vitamins and amino acids are also required for bacterial growth.
Dominguez-Benetton et al. (2018) summarize the general routes through which microbes can catalyze or support metal recovery, leading to nano and macro-scale materials. Competing sorption and electrochemical technologies are briefly revisited. The relevant sources of metals are highlighted, as are the challenges and opportunities to turn microbial electrometallurgy into a sustainable industrial technology in the near future.
Bryant (1987) reviews the progress in enhanced oil recovery using microorganisms, whether in situ or on the surface, and reports on field tests in situ process.
Dwivedi and Soni (2011) provide the importance of soil microbial biomass in derelict mined ecosystems and suggest that an increase in microbial biomass in the soil may enhance soil fertility and provide an effective substrate for nutrient mineralization.
Park et al. (2019) discuss advances in AMD prevention techniques like oxygen barriers, utilization of bactericides, co-disposal and blending, and passivation of sulphide minerals. In addition, recycling mine tailings as construction and geo-polymer materials can reduce the amount of waste for disposal.
Agate (1996) directs the metal extraction processes using microorganisms, which are currently in active use and concern copper and uranium bioleaching. Biobeneficiation is also applied at an industrial scale for the recovery of gold from arsenopyrites. The developments in these processes during the last 15 years, with particular reference to developing nations, are reviewed.
Microorganisms are microscopic organisms that exist as unicellular, multicellular, or cell clusters. Microorganisms are widespread in nature and are beneficial to life, but some can cause serious harm. The growth rate of microorganisms gets influenced by temperature, osmotic pressure, acidity or alkalinity, and oxygen concentration as well. These factors can otherwise be termed environmental factors.
Temperature plays a key role in microbiological processes. In low temperatures (less than 10 degrees), the reaction rate decreases by 50%. For temperatures above 10 degrees, an average sulphate reduction rate of 0.3 mol/m3 of nutrients per day was found where agricultural wastes were used as nutrients (Kuyucak & St-Germain, 1994).
In several in-situ mining operations, production decreases significantly due to the excessive accumulation of bacteria on well screens, casings, and submersible pumps. It is shown that Bacillus spp (All of the species of Bacillus) and pseudomonads can grow at oxygen concentrations of 35 micrograms/ml. However, this problem is resolved with the use of hydrogen peroxide. The addition of hydrogen peroxide was found to inhibit the growth of thiobacilli (Brierley, 1979). At the same time, hydrogen peroxide degrades to produce oxygen that becomes toxic to microorganisms at high concentrations. In general, the high concentration of hyperbaric oxygen inhibits the growth of microorganisms such as pseudomonads and Bacillus sp. in the in-situ leaching environment.
The SRB can be used as a cost-effective alternative to the conventional lime neutralization process for treating ARD. This technique can be used in wetland systems and in bioreactors operated under controlled conditions. The SRB-based passive processes are capable enough to treat seepage, small-contaminated streams, and leached water (acidic in nature) accumulated in open pits after the removal of ores or closure of mines.
Microbes play an important role in commercial mining operations, especially for copper, uranium, gold, etc. In a direct attack, microbial metabolism modifies the redox state of the metal being harvested, increasing its solubility. The other way out is oxidation or reduction of the desired metal followed by solubilization. In gold mining, the use of cyanide solutions is a common practice. As a result, the use of bacteria cells for waste cyanide solution detoxification is encouraged. SRB, also known as sulphate reducers (Desulphovibrio sp.), break down sulphate compounds in acid rock drainage to produce bicarbonate (HCO3-).
This entire process happens in the presence of organic i.e., sulphate carbon sources. Organic carbon, a nutrient, is used as an electron donor in reducing environmental conditions. The significance of anoxic condition lies in the fact that it will slow down sulphur-oxidizing bacteria's performance and enhance Sulfate Reducing Bacteria (SRB) activities, which could successfully prevent and treat ARD. The entire process gets accomplished in 3 stages, i.e. sulphate reduced to hydrogen sulphide (HS-), followed by a reaction between HS- and free hydrogen ions (H+), which generates hydrogen sulphide (H2S). Three 2 different steps are used to summarize the performance of SRBs.
SRB helps convert sulphate to hydrogen sulphide and organic matter to hydrogen carbonate. Toxic heavy metal ions are present to react with the hydrogen sulphide gas to form insoluble metal sulphide precipitates. Sulfide precipitation helps in metal removal. In the last stage, hydrogen sulphide generated insoluble metal complexes, causing the removal of metals.
The SRB process gained popularity because of its ease of availability. This group of bacteria is prevalent in natural soil, sewage products, and manure. Because the process occurs in organic carbon sources or nutrients, they require low-molecular-weight organic carbon compounds. The low-molecular-weight organic compound should be present at sulphate concentrations greater than 200 mg/L and a pH greater than 4.5. The SRB process happens in the presence of organic carbon sources and nutrients. Depending on cost and ease of availability, numerous materials can be used as nutrients.
Aside from SRB, some macrophytes have proven to be effective in dealing with uranium mine waste. During the growth and decay period, the algae act as a filter for contaminants like radium (Ra 226) and uranium.
Biosorption stands for the removal of metal ions from aquatic solutions. Adsorption and absorption occur concurrently (Johnson & Hallberg, 2005). Only by introducing a biosorbent, primarily composed of biological materials, can disposal be made possible. The terms "biosorption" and "bio-accumulation" symbolize the removal of metals by non-living and living cells, respectively. Bioaccumulation involves the transfer of metal from a contaminated matrix to biomass (Phillips et al., 1995). The rasping, toxic conditions resulting from mining do not produce a hospitable state for humans to live in. On the contrary, this condition is conductive for some microorganisms' growth. These microbes derive energy from oxidation-reduction in this toxic slurry, the highly acidic sludge. This oxidation-reduction process helps in releasing economically valuable metals from minerals (Rambabu et al., 2020). It draws the attention of researchers, and the concept of bio-mining and bioleaching originated (Rambabu et al., 2020). In biomining, bacteria are encouraged to extract metal from virgin rock and an abandoned mine (which became a toxic pool). The acid-friendly microbes, or acidophiles, were discovered in 1960, and the concept of biomining was proposed in the 1950s and 1960s (Natrajan, 2006).
Using the bio-mining technique, 10 to 15% of gold mining is done (Schippers et al., 2013). Two processes used in bioleaching are:
In the indirect method, bacteria produce a potent oxidizing agent, reacting with metals and facilitating extraction from ores, as shown in Figure 2. On the contrary, an indirect bioleaching enzymatic attack by the microorganisms takes place.
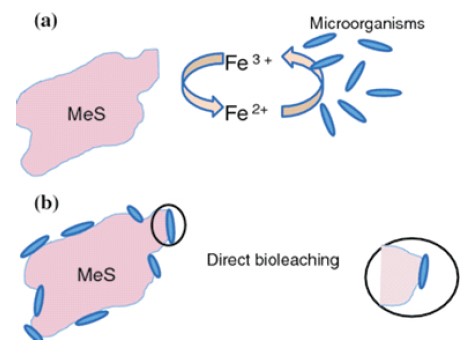
Figure 2. (a) Indirect Process (b) Direct Bio-leaching Process
Not only uranium, but bioleaching is considered a suitable alternative for the extraction of valuable metals from low-grade and waste rocks, i.e., overburden. Microorganisms participate in the leaching process both directly and indirectly. Due to oxidation or reduction, a change in the metal's ionic state happens in a direct attack. In an indirect attack, insoluble metal compounds' transformation into soluble products happens due to organic or inorganic production acids. Bioleaching is extensively used in the recovery of nonferrous metals and the beneficiation of gold-bearing ores. The bacteria Aspergillus niger facilitate the bioleaching of metals by reducing highly oxidized metals (Biswas et al., 2013). Cu from its oxide ores is easy to produce, and the Cu content in them was over 5%. There is a shift from oxides to sulfuric acid copper ores, considering the scarcity of oxides.
In the current scenario, considering the scarcity of oxide ores, sulphide ore minerals are in focus. The problem lies in the negligible copper content, i.e., 1%. Considering the crunch and the available new technique, ore recovery is possible even in very low-grade ores (containing less than 5% of Cu). These ores were not worth mining using conventional mining techniques. Considering current demand, copper ores with 0.3% Cu and slurries with 0.4% or 0.5% Cu are under scrutiny. The nature of metal sulphide determines the rate of bioleaching. In general, chalcopyrite and enargite (primary ores of Cu) are much more difficult to solubilize than chalcocite, covellite, and bornite (secondary ores) (Schippers et al., 2013). For copper leaching, chemolithoautotrophic (acidophilic) is the most commonly used microorganism, as shown in Figure 3.

Figure 3. (a) Copper Mine in Chile Hosts a Bio-mining Operation (b) Acid-Loving Algae (Green) on Copper Sulfide in Black Rock (Everts, 2012)
For energy requirements, they either use ferrous iron or reduced sulphur compounds. The host itself fulfils the chemical requirement, i.e., copper ores. Microbial action results in ferric ion generation, a strong oxidant of copper sulphide that liberates metal into solution. Reduced sulphur compounds get oxidized to sulfuric acid at low pH. This acidic environment promotes acidophiles and the solubility of ferric iron (Natrajan, 2006; Schippers et al., 2013; Biswas et al., 2013). Micro-organisms need nutrients for their growth, and most commonly, they are available in the same environment (Gentina & Acevedo, 2013). Ores and leaching solutions are the sources of nutrients, but they lack nitrogen and phosphorus. This may affect the bioleaching process (Hille et al., 2009). These may need to be added to the leaching solution if economically feasible. Along with these, microorganisms require carbon dioxide (a carbon source) and oxygen to perform carbon source and electron acceptor functions. These two gases are to be transferred from the gas phase to the leaching solution.
Along with nutrients, particle size also affects the leaching rate and efficiency. The degree of sulphide oxidation depends on the specific surface area exposed to the leaching solution (Acevedo & Gentina, 1989). The mining industry already uses prokaryotic organisms. Continued research in this field resulted in the discovery of new microorganisms. Those microorganisms stimulate the growth of bacteria, which extract copper based on lixiviation. According to the results obtained from this investigation, these organisms allow accelerated copper extraction from 1% to 8%.
Microorganisms can be used to extract a specific metal without disturbing the surrounding environment and can help to reduce air and water pollution caused by traditional beneficiation processes. However, the disadvantage is that the process is quite slow and material recovery takes a long time, which can be accomplished in a shorter time frame using conventional methods. Bio-mining is in the development phase and requires the establishment of new infrastructure to make the process more economical than the existing method of beneficiation. Therefore, the mining industry will take years to adopt this concept.
In beneficiation operations, microorganisms cannot be used for a variety of ores. Adding to it, the by-product of bio-mining leads to acid generation, which is a threat to the environment, so proper disposal is warranted. There is no versatile microorganism culture that can be applied universally. Different trace metals, minerals, and their concentrations are guided by the geology of that area and other accessory factors. This indicates each situation requires a unique mixture of microbes for optimum recovery in bio-mining. Further, bio-mining requires new infrastructure, whereas conventional ways (crushing, milling rock followed by flotation, and smelting) are more economical.
Improper waste management results in intense environmental damage in the mining industry. To reduce this ecological damage, suitable remedial strategies are required. Micro-organisms are involved in active mining during unconventional mining. Their presence could impede mining operations. In other instances, their role is vital as their activities cause damage to the environment. At the same time, it also helps mining industries minimize the detrimental effect of Acid Mine Drainage (AMD) and Acid Rock Drainage (ARD) and recover significant metal values from waste, thereby supporting and augmenting natural resources. Bioremediation/biological treatment is a cost-effective and environmentally friendly approach for AMD/ARD pollution reduction. Considering all the pros and cons, one method is not sufficient to curb the issues that are too prevalent on site. It is always preferred or advised to go for a combined passive system to yield better results in preventing and recovering heavy metals. However, further research is still needed to pin down suitable sulphate-reducing microbes and suitable conditions in which maximum metal recovery can be achieved.