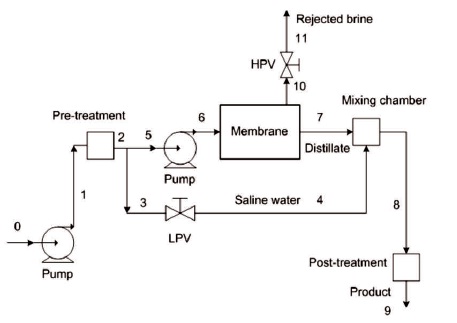
Figure 1. A Schematic of the RO Plant
Two parameters might affect the thermodynamic performance of Reverse Osmosis (RO) desalination plants, those are the recovery ratio and feed water salinity. Exergy analysis is performed to determine the effect of those parameters on the thermodynamic performance of a reverse osmosis desalination unit. Irreversibility, effectiveness, and specific energy consumption are obtained at different recovery ratios and salinities.
The results of the developed thermodynamic model of the present work are validated against the obtained results from the literature, where the effectiveness and the contributions of the membrane, high-pressure valves, friction, and the other components to total irreversibility are compared.
The results show that the contribution of the high valve and membranes to total irreversibility depends strongly on the recovery ratio. The contribution of other components to total irreversibility is a minor one.
The effect of source salinity on the percentage of the recovered exergy is not substantial, for instance, it is found that 7.96% and 6.88% of the destroyed exergy can be recovered, at salinities of 1000 ppm and 5000 ppm, repectively.
The analysis shows that using the Pelton wheel to recover part of the destroyed exergy is only reasonable at low and moderate recovery ratio. For instance, the input power decreases by 7% and 60% for recovery ratio of 0.9 and 0.1, respectively
Reverse Osmosis (RO) is a membrane separation practice that recovers water from a salty solution pressurized to a point bigger than the osmotic pressure of the solution. In principle, the membrane screens out the salt ions from the pressurized solution, permitting only the water to permit. The percentage of RO desalination installations has been growing progressively over the last decade due to the outstanding developments in membrane separation and energy recovery technologies, as well as the linked reductions of overall water production costs (Cipollina, Micale, & Rizzuti, 2009).
The thermodynamic performance of RO has been handled by many authors. An overview of systems engineering approaches for a large-scale seawater desalination plant with a reverse osmosis network was conducted (Kim et al., 2009). In their work, over 100 papers were reviewed to reveal factors influencing largescale seawater desalination plants with reverse osmosis networks.
A thermodynamic study was performed on a Reverse Osmosis (RO) desalination unit with and without energy recovery (Orfi & Salim, 2012). Three configurations of the desalination unit were considered. The first configuration included a throttling valve in the rejection of the concentrated brine side while the two others incorporated a hydraulic turbine and a Pressure Exchanger System (PES), respectively. The results show that the importance of incorporating an energy recovery device when the feed salinity is high.
Thermodynamic analysis of osmotic energy recovery at a reverse osmosis desalination plant was introduced ( ). They evaluated osmotic energy recovery in a seawater RO plant that included state-of-the-art RO membranes, plant designs, operating conditions, and hydraulic energy recovery technology.
The second-law analysis of a reverse osmosis plant in Jordan is performed (Aljundi, 2009). The Reverse Osmosis (RO) plant of Al-Hussein thermal power station was analyzed thermodynamically using actual plant data. The results showed that throttling valves contributed to the highest exergy destruction (56.8%), followed by the exergy destruction in the two-stage RO units (about 21%), the exergy destruction in the pumps and motors were 19.6% in total. The second law efficiency of the plant was very low (4.1%).
A seawater reverse osmosis desalination plant with various energy recovery systems was studied using exergy analysis (Ahmed & Zubair, 2016). The energy recovery devices include turbines and pressure exchangers as well as infinite area based single and two-stage pressure retarded osmosis units. The effect of pump and turbine efficiency, salinity, temperature, and mass ratio were studied.
Exergetic analysis of a reverse osmosis desalination unit was performed for brackish water feed using different energy recovery methods analysis (Ahmed & Zubair, 2015). The analysis included single and two-stage pressure retarded osmosis units. The effect of salinity, turbine and pump efficiency as well as the mass ratio were studied. In all cases, it was seen that the reverse osmosis unit had the best efficiency when a pressure exchanger was incorporated as an energy recovery device.
The thermodynamic analysis was applied to assess the energy efficiency of hybrid desalination cycles that were driven by simultaneous mixed inputs, including heat, electrical work, and chemical energy (Banchik, 2012).
Using real data, the exergetic efficiency was assessed as a performance evaluation tool for a desalination plant located in Gran Canaria -Canary Islands in Spain (Blanco-Marigorta et al.,2017). They pointed out that the components with operation defects should be repaired.
The exergy analysis of a 7250 m3/d Reverse Osmosis (RO) desalination plant in California was conducted by using actual plant operation data, and an alternative design was investigated to improve its performance (Cerci, 2002). It was found that the largest exergy destruction occurred in the membrane modules, and this amounted to 74.07% of the total exergy input. The second law of efficiency of the plant was obtained as 4.3%.
Quantifying the contribution of the various factors to energy consumption in Reverse Osmosis (RO) desalination processes and identify those with the greatest potential for reduction was conducted (Karabelas, Koutsou, Kostoglou, & Sioutopoulos, 2017). Comparative assessment of the results was enlightening, showing that the greatest margin for the desired specific energy consumption reduction is related to improvements in membrane permeability and efficiency of pumps and energy recovery devices.
A second law analysis of a reverse osmosis desalination plant was carried out using reliable seawater exergy formulation instead of a common model in the literature that represents seawater as an ideal mixture of liquid water and solid sodium chloride (Sharqawy, Zubair, & Lienhard, 2011). The analysis was performed using reverse osmosis desalination plant data and compared with results previously published using the ideal mixture model. It was demonstrated that the previous model had serious shortcomings, particularly with regard to the calculation of the seawater flow exergy, the minimum work of separation, and the second law efficiency.
An analysis from first principles for reducing specific energy consumption in reverse osmosis water desalination was performed (Li, 2011). The mathematical model for the performance prediction of a Multi-effect Desalination System with Thermal Vapor Compression (MED-TVC) and a Reverse Osmosis (RO) was presented (Sadri, Ameri, & Khoshkhoo, 2017). The determination of the best tradeoff between the exergetic efficiencies of MED and RO was the final goal of the optimization. The optimum design led to the selection of a MED-RO hybrid system with the highest exergetic efficiency.
Thermoeconomic analysis has been adopted by many authors for performance evaluation of RO desalination plants. The performance of a reverse osmosis desalination plant at different seawater salinity values was investigated (El-Emam & Dincer, 2014). The effects of the system components irreversibility on the economics and cost of product water were parametrically studied through the thermoeconomic analysis. Both thermodynamic and thermoeconomic performances of the overall system were investigated under different operating parameters.
Exergetic and economic analysis of two-pass RO desalination proposed plant for domestic water and irrigation were developed (Eshoul, Agnew, Anderson, & Atab,2017). The analysis covered the influence of recovery ratio on different two-pass reverse osmosis desalination configurations at different seawater temperature and salinity, focusing on power consumption, cost, exergy efficiency, and exergy destruction for a proposed 24000 m3/day two-pass reverse osmosis desalination plant in Libya. The results showed that as the recovery ratio increases, the exergy destruction decreases and exergy efficiency increases, with a slight decline in the cost of the cubic meter with sea water salinity increases.
The evaluation of a preliminary techno-economic of coupling a low-enthalpy geothermal resource and a suitable desalination technology was developed (Loutatidou & Arafat, 2015). The desalination processes were chosen, Multiple Effect Distillation (MED) and reverse Osmosis (RO) were designed as integrated energy-water systems and were compared and assessed in terms of their levelized cost of water produced. It was found that geothermal RO could potentially be a more cost-effective option for seawater geothermal desalination in the Gulf cooperation council countries.
It is already well known that the high-pressure valves and the membrane have the most contribution to exergy destruction in Reverse Osmosis desalination plants. However, the variations of recovery ratio and salinity, which might have a substantial impact on exergy destruction are not well highlighted in the literature. In this work, this impact will be highlighted and quantified.
The flow diagram of the plant is shown in Figure 1. Only sodium chloride is presented in the brine mixture, with a molecular weight of 58.5 kg/kmol. The brackish underground water with a flow rate of 150 kg/s at 101.325 kPa is first withdrawn and pumped by low pressure pumps to 400 kPa. Saline water is pretreated and is divided into two streams: the blend and the process water.

Figure 1. A Schematic of the RO Plant
The process water enters into the high-pressure pump at 385 kPa and leaves at 2200 kPa. Next, the process water is then channeled into the membrane modules and separated into distillate and brine. While the distillate emerges from the membrane modules at a salinity of 50 ppm and a pressure of 101.325 kPa. The brine leaves the membrane at 1600 kPa and passes through High Presure Valve (HPV) through which the pressure is reduced to 101.325 kPa. The distillate, on the other hand, enters into the mixing chamber in which it is mixed with the blend through the Low Pressure Valve (LPV) to produce the product water at 250 ppm. After pre-treatment potable water at atmospheric pressure and temperature is produced.
Saline water is a mixture of salt and pure water. Salinity is expressed in parts per million (ppm), and related to the mass fraction of the solid particles x as (Cerci, 2002):
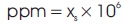
Solutions that have a concentration of less than 5% are considered to be dilute solutions. Dilute solutions closely approximate the behavior of an ideal solution, and thus the effect of dissimilar molecules on each other is negligible. Saline underground water and even sea waters are all ideal solutions since they have about a 4% salinity at most (Cerci, 2002).
The mass of the mixture (m) is:
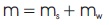
where ms and mw are the mass fractions of solid and water, respectively.
Dividing by the mass of the mixture, we obtain:

where xs and xw are the mass fraction of solids and water, respectively.
Also, the number of moles of a mixture (N) is:
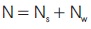
where Ns and Nw are the number of moles of solid and water, respectively.
Dividing by the number of moles of the mixture, we obtain:
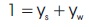
where ys and yw are the mole fractions of solid and water, respectively.
Also, we may write:
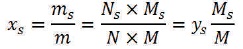

where M, Ms, and Mw are the molecular weights of mixture, solid, and water, respectively
where,
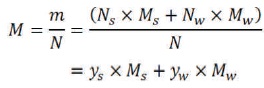
Then the mass and mole fraction can be related as,
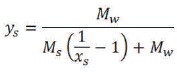
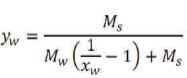
Total exergy  is the summation of the physical exergy
is the summation of the physical exergy  and chemical exergy
and chemical exergy 
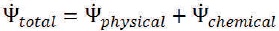
The physical exergy is given as (Wark & Junior, 1994):
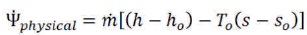
where h and s are the enthalpy and entropy. The subscript “o” refers to the environmental condition.
Also the intensive thermodynamic properties are related as (Wark, 1994):
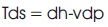
where T, v, and p, are the temperature, specific volume, and pressure, respectively. If T is constant and equal to To, then equation (12) can be approximated as,
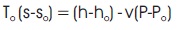
In this case, the physical exergy becomes:
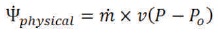
The chemical exergy is expressed as (Wark, 1994):
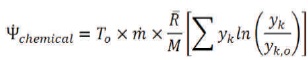
R is the universal gas constant:
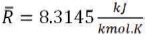
The subscript k refers to the component k.
The effectiveness of the plant (e) is calculated as (Wark, 1994):
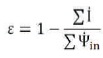
Í is the rate of the irreversibility in kW.
For the system under consideration (Figure 1), the Recovery Ratio (RR) is defined as:
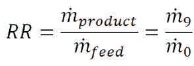
Knowing the feed flow rate and the Recovery Ratio (RR), the product flow rate can be calculated, where the mass balance implies (Figure 1):
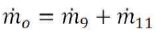
And for the solid:
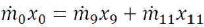
Then, we may have,
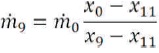
Knowing the salinities of the product (x9) and feed water (x0), then the salinity of the rejected brine (x11) can be found form equation (20).
The mass flow rate of the rejected brine can be written as,
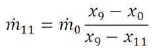
Mass balance on the mixing chamber would give
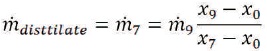
The extracted water flow rate to the mixing chamber is,
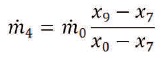
Using the foregoing equations, the exergy per unit mass and exergy flow rates at different points designated in Figure 1 is evaluated. When exergy flow rates are available, the destroyed exergy within any component can be calculated from exergy balance equations. Note that the exergy of raw brackish water is zero since its state is taken as the environmental dead state. The brackish water emerges into the system at the environmental dead state and the leaving streams of the disposal brine and product water departure the system at the restricted dead state, with different salinities. Consequently, the exergies of the leaving streams present the chemical exergies and differ due to salinities differences.
The results of the developed thermodynamic model of the present work are validated against the obtained results from (Cerci, 2002), where the effectiveness and the contributions of the membrane, High-pressure Valves (HPV), friction, and the other components to total irreversibility are compared. The result of the validation is shown in Table 1. Excellent agreement is obtained.

Table 1. Model Validation, Irreversibility %, and Effectiveness
For the analysis, the dead state is selected as P0 = 101.325 kPa, and T0 = 288.15 K and salinity of 1000 ppm (Ahmed & 0 Zubair, 2015).
To explore the effect of the recovery ratio, the salinity of the brackish water is kept constant at 3500 ppm, while the recovery ratio varies between 0.10 and 0.90.
Figure 2 shows the irreversibility of the membrane, high-pressure valves, and other components as a function of the recovery ratio. The reduction in the high-pressure valve irreversibility as shown is due to the reduction in the mass flow rate of the disposal brine with the increase in the recovery ratio. Furthermore, both the flow rate of the distillate water and hence the exergy destruction across the membrane increases with the recovery ratio.
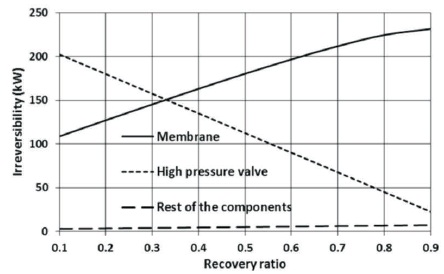
Figure 2. The Effect of Recovery Ratio on Irreversibility
The results show that the contribution of the high valve and membranes to total irreversibility depends strongly on the recovery ratio. The contribution of other components to total irreversibility is a minor one.
Different authors reported different percentage of the membrane's contribution to total irreversibility, values between 21 and 74% have been reported (Ahmed & Zubair, 2015). In the present work, depending on the recovery ratio, the contribution of the membrane to total irreversibility varies between 34.63 and 88.70%, while the contribution of the high-pressure valves varies in the range of 64.48 and 8.61%. Both, membrane module and high-pressure valve contribute to about 49% of the total irreversibility at a recovery ratio of about 0.330, as shown in Figure 3.
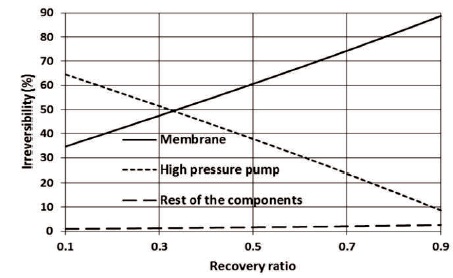
Figure 3. The Effect of the Recovery Ratio on the Irreversibility Percentage
The analysis shows as the recovery ratio increases, more saline water is blended for the mixing process in order to reach the desired salinity of the product water. As a result, a dropping in the input power of the high-pressure pump is noticed as shown in Figure 4. As can be seen, the rate of reduction of the irreversibility is higher than that of the input reversible power.
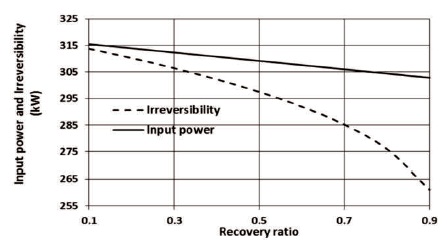
Figure 4. The Effect of Recovery Ratio on Input Power of the High-Pressure Pump and Irreversibility
Accordingly, the effectiveness of the plant increases with the increase in the recovery ratio as shown in Figure 5. The results are in good agreement with that given in (Eshoul et al., 2017).
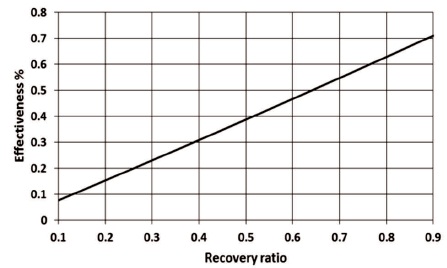
Figure 5. The Effect of Recovery Ratio on the Effectiveness
Membrane module accounts for the large portion of the exergy destruction for reasonably high recovery ratio. However, maybe nothing much to do to eliminate or reduce this lost exergy economically, as far as membrane technologies are concerned (Cerci, 2002). Other exergy destruction that arises throughout the system can be considered to be minimal compared to the exergy destruction during the separation, and thus they do not worth much consideration.
The most reasonable and practical way to increase the effectiveness or decrease the power input of the plant considerably seems to be by substituting the throttling valve on the brine stream by a pressure exchanger. Using the Pelton wheel to recover 94% of the destroyed exergy in the high-pressure valve reduces the specific energy consumption, this can be shown in Figure 6. However, the results show that this practice is reasonable for low or moderate recovery ratio. For instance, for recovery ratio equal to 0.90, the specific input exergy decreases by 7%, while the specific input exergy can be decreased by 60% for 0.10 recovery ratio.
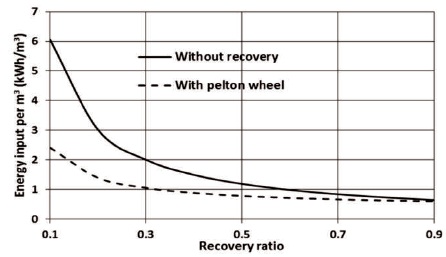
Figure 6. The Effect of Recovery Ratio on the Specific Energy Consumption
To reveal the effect of salinity on the thermodynamic performance of RO plant, the recovery ratio is kept at 0.90 and the salinity of the source brackish water is varied between 1000 and 5000 ppm, other properties are kept constant. Figure 7 shows the effect of the salinity on the irreversibility. As can be seen, the contribution of the membrane to total irreversibility is the highest for relatively high recovery ratio (0.90) since the flow rate through the high-pressure valve is relatively small. The contribution of the rest of the components is not weighty.
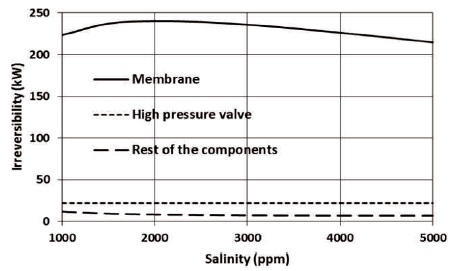
Figure 7. The Effect of Salinity on Irreversibility
With the increase of salinity of the source water, the mass flow rate of saline water through the high-pressure pump also increases causing an increase in the input power of the high-pressure pump, which can be shown in Figure 8. The effect of the salinity on the total irreversibility of the plant is also depicted in Figure 8.
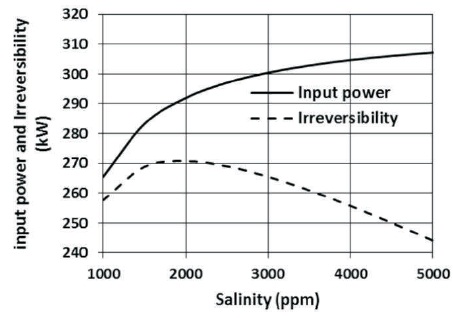
Figure 8. The Effect of Salinity on Input Power of the High-Pressure Pump and Irreversibilities
The salinity and hence the chemical exergy of the rejected water increases significantly with the increase in the salinity of the source brackish water. For instance, for brackish water salinity of 1000 ppm, the salinity of the rejected stream is 7,750 ppm and the chemical exergy is 5.6261 kW, while for brackish water salinity of 5000 ppm, the salinity of the rejected stream is 47,750 ppm, and the chemical exergy is 85.8444 kW. The significant rise in the exergy of the rejected water causes a reduction the effectiveness of the plant as shown in Figure 9.
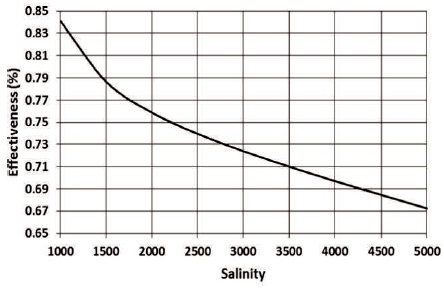
Figure 9. The Effect of Salinity on the Effectiveness
For constant recovery factor (0.9 in this case), the amount of the recovered water is constant, for this case, the rate of the recovered water is 135 kg/s (90% of the input brackish water). Therefore, the increase in the input power with the increase in salinity of the source water, causes an increase in the specific input power as shown in Figure10. Using the Pelton wheel to recover 94% of the destroyed exergy in the high-pressure valve, reduces the specific input exergy.
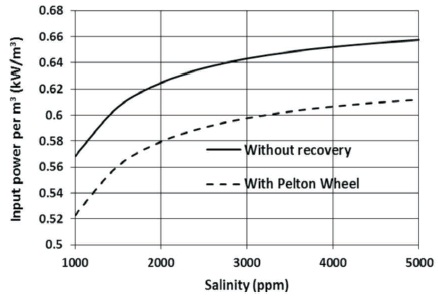
Figure 10. The Effect of Salinity on the Specific Input Power
The results show that the effect of source salinity on the percentage of the recovered exergy is not substantial. For instance, at a salinity of 1000 ppm, it is found that 7.96% of the destroyed exergy can be recovered, while at a salinity of 5000 ppm, it is found that 6.88% of the destroyed exergy can be recovered.
Exergy analysis is performed to find the effects of recovery ratio and brackish water salinity on the thermodynamic performance of a reverse osmosis desalination plant. The analysis shows that: