
The main objective of this research was to examine the corrosive inhibition property of 1,1-biss-2-Naphthol on the aluminum surface in 3 M HCl environment by weight loss, gasometric, Tafel plot, and impedance spectroscopy techniques. Weight loss results show that addition of1,1-biss-2-Naphthol to the examined solution significantly increases the protection efficiency (decreases dissolution rate). Gasometric studies show that addition of 1, 1-biss-2-Naphthol to the aggressive acid solution effects on the rate of evolution of hydrogen gas. The Tafel plot results show that the synthesized compound 1, 1-biss-2-Naphthol behave as mixed type and hinders cathodic aluminum and anodic aluminum reactions. Impedance studies show that enhancement in the depressed semi circle is due to the positive effect of synthesized compound 1, 1-biss-2-Naphthol.
Al is one of the major materials used in different industries because of its noble chemical and physical characteristics. During closed-loop systems and several batch operations in industries, Al metal comes in contact with the hydrochloric acid solutions. HCl was used for the pickling, cleaning, petrochemical, and oil well acidizing processes. The interaction between the Al metal and hydrochloric acid solution at higher temperature (usually at 60 0C) leads to the dissolution or disintegration of the aluminum metal. Dissolution or disintegration of the Al metal is called corrosion. The Al corrosion has great economical and environmental impact due to its wide spread applications in the different industries. Consequently, great research effort has been devoted to discover the new appropriate corrosion inhibitor for the Al in harsh corrosion environment (James, Oforka, & Abiola, 2006; Subramanyam, Sheshadri, & Mayanna, 1993; Obot, Obi-egbedi, & Umoren, 2009). Hence, prevention of Al corrosion in the harsh corrosive system is vital. The use of coatings, inhibitors, cathodic protection, anodic protection, and corrosion resistance material are five practical techniques used for the prevention of corrosion of Al. The use and application of corrosion inhibitors was verified to be the most efficient, economical, and practical method in protecting the surface of Al against corrosion (Zhang & Hua, 2010; Ashassi-sorkhabi, Shabani, Aligholipour, & Seifzadeh, 2006; Rosaliza, Wan nik, & Senin, 2008; Fares, Maayta, & Al-Qudah, 2012; Fouda, Abu-El-Nader, Moussa, & Shehata,1988). Corrosion inhibitors are chemical components (which possess electron rich elements N, S, O, and P) and when introduced into the acid solution, blocks the aluminum dissolution or disintegration process. Many scientists (especially corrosion scientists) report the corrosion inhibition property of several organic compounds on the Al electrode in the HCl medium. Yet the majority of organic species are expensive, and the synthesis of organic compounds involves multiple steps, which take time (Khadraoui, Khelifa, Hachama, & Mehdaoui, 2016; Abiola & Tobun, 2010; Abdallah, 2004; Abiola & Otaigbe, 2009). This research selected simple route for the synthesis of an organic compound in order to examine corrosion inhibition property on the Al surface in HCl system. In the present investigation, the compound 1,1-biss-2-Naphthol is synthesized through simple procedure and corrosion inhibition of 1,1-biss-2-Naphthol was systematically examined through weight loss, gasometric, Tafel plot, and impedance spectroscopy techniques.
The weight composition of Al (in weight percentage) was as follows: 0.1% Cu, 0.4-0.9% Mg, 0.2% Cr, 0.3% Mn, 0.2% Zn, 0.1% Tl, 0.3-0.7% Si, 0.6% Fe, and 96.9-97.8% Al. Surface treatment of the Al involves with different grade sand papers. Bristle brush is used to remove the corrosion products on the Al surface. The inhibitor is prepared as follows:
2- naphthol (10 g) and 100 ml of double distilled water in a 250 ml conical flask were taken and heated until the mixture starts to boil. Then, 15 g of aqueous ferric chloride is added to the hot solution. The reaction is carried out for one hour and filtration is carried out by using suction pump. The resulting sample (1, 1-biss-2-Naphthol) is crystallized by toluene. The melting point of the resulting compound is 215 0C [Scheme 1]. The inhibitor of four different amounts, i.e. 0.1 g/L, 0.2 g/L, 0.3 g/L, and 0.4 g/L is prepared. The 3 M HCl environment was prepared from the analytical grade HCl and double distilled water. Weight loss and gasometric studies were carried out with 100 ml of 3 M HCl system containing 3 mg/L, 6 mg/L, 9 mg/L, and 12 mg/L of 1,1-biss-2-Naphthol at 60 0C. The aluminum metal pieces are completely submerged in the 3 M HCl solution. After 1, 2, 3, 4, days, the Al metals are retrieved from the harsh acid environment and weight loss of the Al were recorded.
Weight loss and gasometric studies were carried out three times and the obtained results show good reproducibility. From the Al weight loss values, the protection efficiency of 1,1-biss-2-Naphthol can be calculated by using the following equation,

where, W1 = Al weight loss of MS without 1,1-biss-2- Naphthol, W2 = Al weight loss with 1,1-biss-2-Naphthol.
From the amount of hydrogen gas evolution, the protection efficiency of the 1,1-biss-2-Naphthol can be calculated by the below relation,

where, Va = Amount of H2 gas liberated without 1,1-biss-2-Naphthol, and Vp = Amount of H2 gas liberated with 1,1- biss-2-Naphthol.
Tafel plot and impedance spectroscopy techniques were conducted at 60 0C with Pt (counter cell), calomel (standard), and Al (working) cell by using CHI workstation. The electrochemical experiment was performed in unstirred and aerated solution after stabilization period (approximately 50-60 minutes).
Table 1 shows the weight loss data of Al in 3 M HCl without and with of 1,1-biss-2-Naphthol at 60 0C. From Table 1, it is observed that the corrosion rate of aluminum in 3 M HCl solution with1,1-biss-2-Naphthol decreased and the protection efficiency enhanced progressively with enhancing the inhibitor concentration, which showed that electron rich groups present in the inhibitor 1,1-biss-2- Naphthol gets adsorbed on the Al surface. The reduction in the aluminum corrosion rate in the presence of 1, 1-biss- 2-Naphthol, is due to the positive (inhibitive) effects of 1, 1- biss-2-Naphthol. The maximum protection efficiency of 96.666% was successfully achieved at 12 mg/L of Al for immersion period of 1 day. But, at higher immersion period, the protection rate drastically decreased to 87.704% at 12 mg/L of 1,1-biss-2-Naphthol for four days immersion period. The enhancement in the Al corrosion rate is a complex phenomenon because at higher immersion period, rapid etching, desorption, and decomposition of 1,1-biss-2-Naphthol molecules take place.
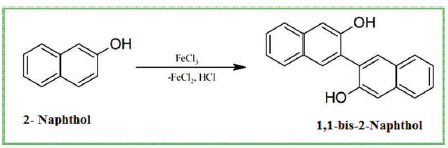
Scheme 1 Preparation of 1,1-biss-2-Naphthol
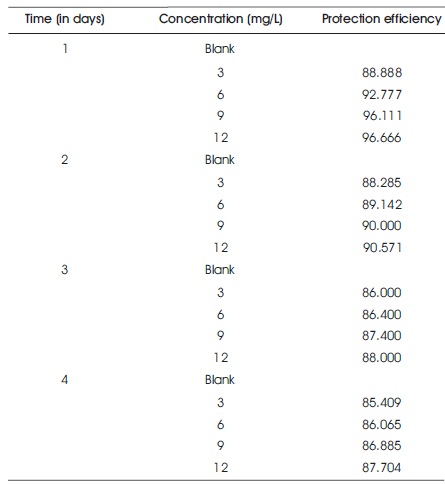
Table 1. Weight Loss Results
To support the weight loss results, gasometric experiment was carried out on the Al in the 3 M HCl solution at 60 0C. This technique provides good information related to the protection efficiency of the inhibitor. The results of gasometric techniques are shown in Table 2. After addition of 1,1-biss-2-Naphthol to the corrosive solution reduces the volume of hydrogen gas evolved. The volume of hydrogen gas evolved further decreases (which is an indication of enhanced protection efficiency) with increase in the amount of 1,1-biss-2-Naphthol from 3 mg/L to 12 mg/L. This trend confirms the inhibition action of 1,1-biss-2-Naphthol on the Al surface in 3 M HCl medium. Low protection efficiency was observed with increase in the time from one hour to ten hours. This is due to the desorption of 1,1-biss-2-Naphthol over the Al surface in the 3 M HCl medium. The results of gasometric results well support the weight loss results.
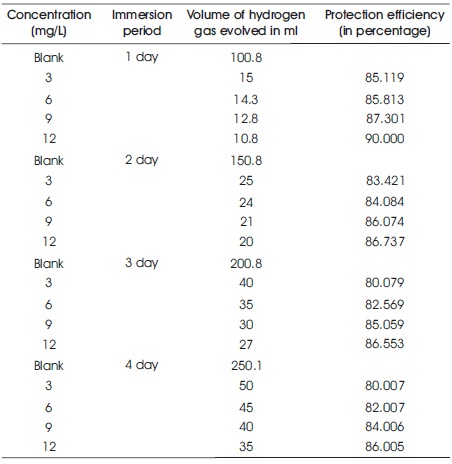
Table 2. Gasometric Results
The relationship between the potential-current for the Al electrode in the 3 M HCl with different amounts of 1, 1-biss- 2-Naphthol are shown in Figure 1. The results of Tafel plots are shown in Table 3. The value of corrosion current density in the bare solution is high compared to the protected systems. This shows that presence of 1, 1-biss-2-Naphthol hinders the disintegration process of Al in the 3 M HCl solution. Presence of inhibitor 1, 1-biss-2-Naphthol cause reduction in the Al corrosion rate, so it moves cathodic and anodic Tafel curves to lower corrosion current density values. This confirms that anodic dissolution and hydrogen evolution are protected. This nature is due to the adsorption of 1, 1-biss-2-Naphthol, on the corroding Al surface. The anodic and cathodic Tafel slope values were not significantly varied towards any direction and also changes in the corrosion potential value is not greater than 85 mV. This shows the mixed corrosion inhibition property (inhibition of both cathodic and anodic reaction) of 1, 1-biss-2-Naphthol, over the surface of Al in the 3 M HCl solution.
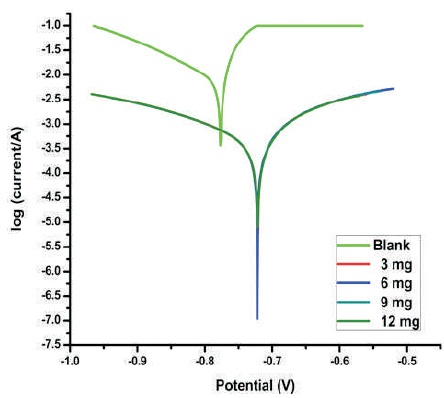
Figure 1. Tafel Plots
Impedance spectroscopy study results are shown in Figure 2 and Table 4. It is observed that the area of depressed semicircle increases with inhibitor concentration of 1, 1-biss-2-Naphthol, which is due to the charge transfer process. The charge transfer resistance is a measure of electron transfer process at the metal corrosive solution interface and it has inverse relationship with the aluminum corrosion rate. The increased charge transfer resistance values with increase in the inhibitor concentration 1, 1-biss-2-Naphthol, is a hint of corrosion inhibition property of 1, 1-biss-2-Naphthol, over the Al surface in the 3 M HCl solution. The protective layer formed by the 1, 1-biss-2-Naphthol, over the Al surface increases the impedance of the surface of the electrode to the electrochemical corrosion. The maximum protection efficiency obtained from the impedance spectroscopy technique is 98. 870%.
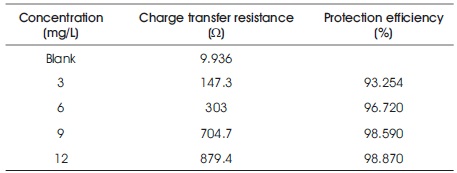
Table 4. Nyquist Plot Results
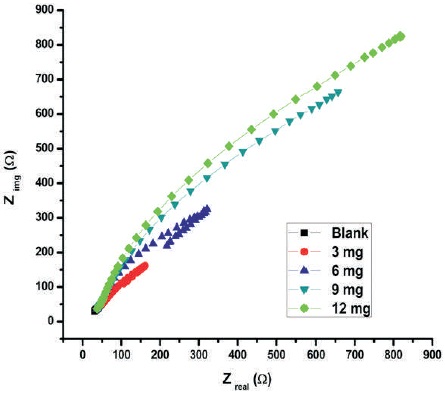
Figure 2. Nyquist Plots
This work presents the corrosion inhibition property of 1, 1- biss-2-Naphthol, over the Al surface in 3 M HCl solution. The compound 1, 1-biss-2-Naphthol can be used as corrosion inhibitor for Al without addition of other compound for Al in 3 M HCl solution at low concentration. An enhance in the time causes a reduction in the protection efficiency values. The corrosion inhibition is achieved by adsorption process. The compound 1, 1- biss-2-Naphthol acts as a mixed corrosion inhibitor. Impedance studies show that the compound 1, 1-biss-2- Naphthol, controls the corrosion process by charge transfer process. The maximum protection rate is observed with one day of immersion time.