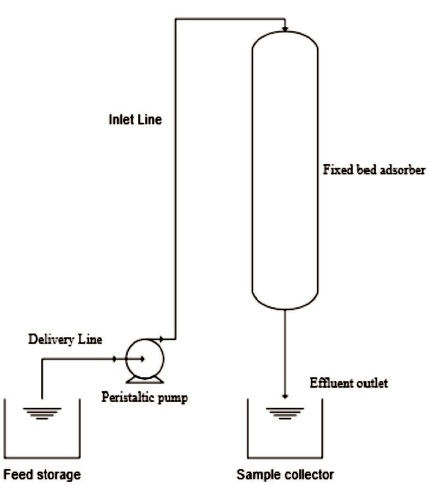
Figure 1. Experimental Set-up
Acetic acid is widely used as an industrial chemical due to its very good solvency and miscibility. It is also used as a reagent in the production of many industrial chemicals. The major use of acetic acid is in the manufacture of vinyl acetate monomer, narrowly followed by acetic anhydride and ester. The total worldwide production of acetic acid is expected to reach 15.5 Mt/a (million tons per year) by 2020. Waste Acetic acid with pH of 5 or lower is termed as a dangerous waste. There are many methods used for removal of acetic acid from aqueous solutions; however these are also energy and cost intensive. Adsorption is one of the alternate methods which can be used for the removal of acetic acid. Considering the economics, there is increasing research interest in using alternate low cost adsorbents. In this work, adsorption of acetic acid from aqueous solutions by using Rice husk has been explored. The adsorption of Acetic acid in continuous mode has been studied with two variables (adsorbent dosage, adsorbate dosage), keeping one constant at a time. Breakthrough curves were obtained. The results showed that the proposed adsorbent is very useful for removing Acetic acid from industrial wastewater.
Adsorption is a method which takes place when a liquid or gas solute builds up on the outside of a liquid or solid (adsorbent), forming an atomic or molecular film (adsorbate). It is dissimilar from absorption, where a material diffuses into a solid or liquid for forming a solution. The word sorption includes both methods, whereas desorption is the turnaround process. Adsorption is operational in a good number of usual chemical, biological, and physical systems, and is extensively used in engineering applications like water purification, synthetic resins, and activated charcoal. Adsorption is an outcome of surface energy similar to surface tension (Guyo et al., 2015; Sousa et al., 2010). In a bulk matter, all the bonding necessities (be they metallic, covalent, or ionic) of the ingredient atoms of the material are completed. However, atoms on the clean surface face bond insufficiency, as they are not completely bounded by other atoms. The precise nature of bonding is dependent on the information of the species concerned, but the adsorbed material is usually classified as displaying chemi-sorption or physi-sorption (Özer, 2007; Velazquez-Jimenez et al., 2013).
Adsorption operations use the capacity of certain solids to concentrate particular material from solutions on their surfaces. In these operations, the mixture to be separated is brought into contact with another insoluble phase, the adsorbing solid and the unequal distribution of the original constituents between the solid surface and the bulk of the fluid permits a separation to be made. The solid is known as adsorbent and the component adsorbed is known as adsorbate. The commonly used adsorbents are: activated carbon, silica gel, activated alumina, etc. (Long et al., 2014; Tchobanologus et al., 2003).
The current work has been carried out to deal with the public problem of acetic acid pollution of industrial waste water. This paper focuses on the studies in the exclusion of acetic acid from wastewater using Rice husk as a low cost adsorbent.
Physical adsorption or Physi-sorption is a sort of adsorption where the adsorbate sticks to the surface due to Van-der- Waals (weak intermolecular) interactions. Chemical adsorption or Chemi-sorption is a sort of adsorption, where a molecule sticks to the surface due to the development of a chemical bond. Quite a few models describing the progression of adsorption, namely Langmuir isotherm, Freundlich isotherm, BET isotherm, etc., are available in literature (Mondal, 2009; Dwivedi et al., 2008).
Water contamination by acetic acid coming from expulsion of wastewater sent out from industries is a key concern. Waste Acetic Acid with pH of 5 or lower is termed as a dangerous waste. Acetic acid present in soil and waterbodies can cause numerous long term repercussions on its biological, chemical, and physical characteristics. Exposure to acetic acid may also be hazardous depending on the concentration. Concentrated acetic acid acts corrosively on the skin. It also gives symptoms of upper respiratory tract irritation, conjunctive irritation, and hyperkeratotic dermatitis. There are also cases of blackening and hyperkeratosis of the skin of the hands, bronchitis, conjunctivitis, and erosion of the exposed teeth (canines and incisors) (Han et al., 2006).
A variety of technologies are available for the eradication of acetic acid. These include membranes, ion exchange, and adsorption with activated carbon. A lot of them are typically expensive. At present, there is a rising consideration in treatment with low-cost adsorbents. Engineers and scientists have developed comparatively low cost adsorbents from biomass, called bio-adsorbents. Adsorbents are extremely proficient materials that have huge prospective to replace usual treatment methods. Considering the cost-effective view point, there is a bulging consideration in using alternate low-cost adsorbents. Lately, rice husk is gaining position due to its potential in removing heavy metals (Sousa et al., 2010; Sastry & Rao, 2017).
The yearly global rice husk amount produced is approximately 80 million tons and above 97% husk is produced in the developing countries. The benefits of utilizing rice husk as an adsorbent is its property of good adsorption and biodegradability, which may be because of its surface functional groups and morphology. Its function got a good deal of consideration in adsorption of different pollutants from the aqueous medium. The rice husk has demonstrated utmost adsorption ability for diverse metal ions in terms of different factors like temperature, initial concentration, contact time, pH, and dosage. Rice husk has also proved helpful in the handling of wastewater having lead and zinc (Goel et al., 2005; Qaiser et al., 2009).
This also finds application in the following areas: Removal of H2O from organic solutions, Removal of sulfur compounds from organic solutions, De-colorization of sugar solutions using bone char in sugar industries, Drying of air in Food industries, Dehydration of gases, Removal of pollutants from effluents, Removal of heavy metals from water. Apart from these it can also be used in Separation of: Normal paraffins from iso-paraffins, Normal paraffins from olefins, P – xylene from other C8 aromatics, p or m – cymene from other cymene isomers, and Fructose/dextrose from polysaccharides (Chen et al., 2012; Lima et al., 2012).
The system consists of Rice husk, Distilled water, and Acetic acid.
The apparatus used are continuous adsorption column, conical flask, burette with stand, funnel, pipette, and measuring jars.
Rice husk is washed with distilled water until the washings are free from color and pH of the solution is 7. It is then dried in the oven at 100 oC for 2 hours. The dried rice husk is subjected to a ball mill and the size is reduced to 200 mesh (Sastry & Rao, 2016).
A schematic diagram of the experimental set-up is shown in Figure 1. Experiments were performed in a glass column of 60 cm height and 3 cm diameter. Experiments were performed by changing the amount of rice husk adsorbent. Feed consisting of acetic acid (0.5, 0.75, 1 liter) and distilled water (9.5, 9.25, 9 liters) is added to Tank-1 and thoroughly mixed using glass rod. The initial concentration of acetic acid Co is found by titration with NaOH. A constant flow rate of the feed from Tank-1 to the adsorbent column is maintained.

Figure 1. Experimental Set-up
The treated samples were collected from the exit stream for every 1 min and the concentration of acetic acid, C* is measured by titration with NaOH. The same is continued for every 1 minute until C* ~ Co (C* is approximately equal to Co, which indicates that the adsorbent bed is saturated with solute). The values are tabulated. The breakthrough curve is drawn between equilibrium concentration of acetic acid in outlet stream, C* vs. time.
Figure 2 shows the relation between adsorption of aqueous acetic acid on the surface of rice husk with varying adsorbate dosage. It is also observed that for 20 gms of adsorbent, as the amount of adsorbate is increased from 500 ml to 1000 ml, the rate of adsorption increases. For the same concentration of filtrate sample (3mg/l approx.) rate of adsorption is high for 1000 ml adsorbate (acetic acid) taken.
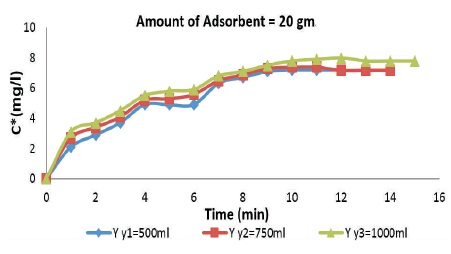
Figure 2. Breakthrough Curves for 20 gm Rice Husk Adsorbent
Figure 3 shows the relation between adsorption of aqueous acetic acid on the surface of rice husk with varying adsorbate dosage. It is observed that the concentration has been increasing till 6 min and after that the bed got saturated and as the value got equal to the initial concentration of the feed. For 40 gm of adsorbent, it is observed that as the amount of adsorbate is increased from 500 ml to 1000 ml, the rate of adsorption increases.
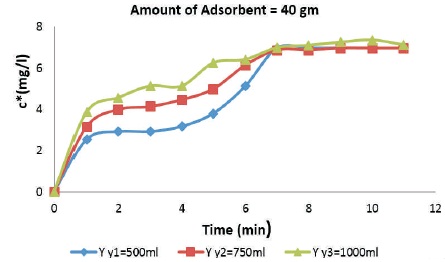
Figure 3. Breakthrough Curves for 40 gm Rice Husk Adsorbent
Figure 4 shows the relation between adsorption of aqueous acetic acid on the surface of rice husk with varying adsorbate dosage. The amount of adsorbate that is being adsorbed is more in this case when compared through the rate of adsorption in case of 20 gm and 40 gm. For 60 gm of adsorbent, it is observed that as the amount of adsorbate is increased from 500 ml to 1000 ml, the rate of adsorption increases.
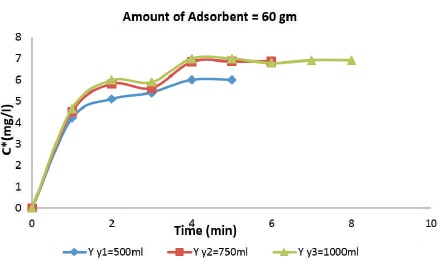
Figure 4. Break through Curves for 60 gm Rice Husk Adsorbent
Table 1 shows that the breakthrough concentration (maximum permissible concentration) increases as the amount of adsorbate increases.

Table 1. Amount of Adsorbate vs Breakthrough Concentration
Figure 5 shows the relation between adsorption of aqueous acetic acid on the surface of rice husk with varying adsorbent dosage. It is inferred that the rate of adsorption is more when amount of rice husk adsorbent is more. The deviation in the values of concentration during 40 gm adsorbent may be due to the flow fluctuations.
Figure 6 shows the relation between adsorption of aqueous acetic acid on the surface of rice husk with varying adsorbent dosage. It is inferred that the rate of adsorption is more when amount of rice husk adsorbent is more.
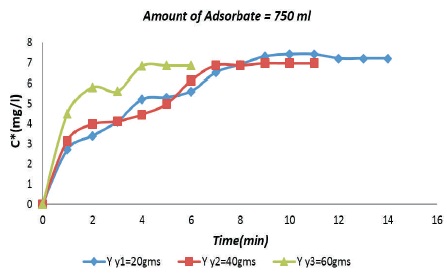
Figure 6. Breakthrough Curves for 750 ml Acetic Acid
Figure 7 shows the relation between adsorption of aqueous acetic acid on the surface of rice husk with varying adsorbent dosage. The deviation in the values of concentration may be due to the flow fluctuations.
Table 2 shows that the equilibrium time decreases as the amount of adsorbent is increased.

Table 2. Amount of Adsorbent vs. Equilibrium Time
This study was performed to explore the use of a fixed-bed adsorption column to treat aqueous acetic acid using an inexpensive adsorbent. Rice husk is capable of removing Acetic acid from aqueous solution. The Acetic acid adsorption performance by Rice husk is strongly affected by parameters, such as amount of acetic acid and amount of rice husk adsorbent. Break-through response curve has been developed for the adsorption of acetic acid on rice husk. Feed flow continues following the event of breakthrough due to which the concentration of solute rises and the breadth of mass transfer zone reduce. Eventually, the rear of mass transfer zone reaches the top of the bed. The whole bed is then said to be saturated, i.e. in equilibrium with the liquid at feed concentration. The time required for this to happen is called “Equilibrium Time, te”. The equilibrium time decreases as the amount of adsorbent is increased. The breakthrough concentration (maximum permissible concentration) increases as the amount of adsorbate increases. Adsorption of Acetic acid is highest for 1000 ml of acetic acid solution.
The adsorbent ability of the bed enhances by increase of adsorbent dosage. Adsorption of Acetic acid is highest for 60 gm of rice husk adsorbent in the bed. Therefore rice husk is a fine adsorbent for the exclusion of aqueous acetic acid.
From the study it is recommended that rice husk can successfully be used for the removal of acetic acid from aqueous solutions. It is a low cost adsorbent and has a lot of potential for purification of industrial wastewater.