 of the corresponding crystals. Shear planes were then bright polished and followed by annealing (in the inert gas atmosphere) at 1500 oC for 60 minutes, and were coated with the heat resistant alloy of Fe-Cr-Al-RE (RE=La or Y) system.
of the corresponding crystals. Shear planes were then bright polished and followed by annealing (in the inert gas atmosphere) at 1500 oC for 60 minutes, and were coated with the heat resistant alloy of Fe-Cr-Al-RE (RE=La or Y) system.Self-healing capability of newly developed Fe45Cr4Al1Ni03La refractory alloy has been investigated in this study. During the tests as substrates, the authors used both monocrystals (Nb and Mo) and polycrystalline (low alloyed Cr) samples. The selected substrates were coated with Fe45Cr4Al1Ni03La refractory alloy using the electron-beam vacuum evaporation technique followed by high temperature (1200 oC) treatment. The structural properties of the Fe45Cr4Al1Ni03La composition was studied using various techniques, such as SEM, WDS, AES, and LM. The results of the experimental studies demonstrated that the Fe45Cr4Al1Ni03La coating layer on the selected substrate surfaces at high temperatures has an ability to heal the micro cracks created as a result of mechanical and thermal damages. It is shown that the Fe45Cr4Al1Ni03La coating composite exhibits high adhesion with all selected substrates, and consequently, a high protective capability against both corrosion and wear at high temperatures. The newly developed Fe45Cr4Al1Ni03La composition is considered as a breakthrough engineered material for commercial applications at high temperatures.
The needs for high temperature construction materials occasionally exceed the potential capacity of traditional heat-resistant nickel, cobalt, and iron base alloys. Therefore, the development of new high temperature corrosion resistant refractory alloys has become one of the important problems of materials engineering. The perseverance of the problem becomes tougher due to the facts that there are basically no commercial materials which would be able to operate for a long time duration at high temperatures (>1200 oC) in the aggressive environment (Birks et al., 2006; Okrosashvili et al., 2009).
Single crystal heat resistant metals and superalloys are commonly used as materials for high pressure blades of gas turbines and jet engines. These materials are used at high temperature applications where creep resistance is one of the crucial factors. Due to their good heat-resistance some refractory metals (Nb, Mo, Ta, Cr, etc.) and their compositions are considered as high temperature structural materials with potentials for high temperature commercial applications (Birks et al., 2006; Fournier, 1995). One of the drawbacks of applications of conventional refractory metals and alloys at high temperatures is their vulnerability to gas corrosion. Therefore, in high temperature applications such as gas turbines and jet engines the oxides Al2O3, SiO2, Cr2O3 are utilized (Padture et al., 2002; Mueller et al., 1992; Cockeram et al., 1995, 1996; Okrosashvili et al., 2009).
Currently, there are no such metallic materials which by the combination of their heat-resistant and oxidation resistant features could provide functional resources of highly efficient, both the air and the land base, more reliable gas turbine engine parts. It is very difficult to obtain optimal composition features using single materials. Thus, in order to resolve the mentioned difficulty, scientists are trying to use such combined composition materials the bases of which are high heat-resistant (high-temperature creep resistant) refractory metals (or metallic alloys), on the surfaces of which it is formed the protective, oxidation resistant coatings with high temperature anticorrosive and thermal-barrier features.
Because of the excellent thermal insulation ability, Thermal Barrier Coatings (TBC) are widely used for advanced gas turbine engines in order to protect key components from very high temperatures. In thermal barrier coating systems for current advanced gas turbine engines, the actual thermal barrier is a ceramic top layer that should satisfy some known basic requirements. However, the most important selection criteria for TBC materials on refractory metals and alloys are to meet the following requirements: 1) high melting point; 2) high chemical inertness and stability at very elevated temperatures; 3) thermal expansion matched with the metallic substrate, 4) adherence to the metallic substrate. Another serious issue in combined compositions coatings is the mechanical bonding strength between the coating layers and the metallic substrate as cracking and peeling of the coating layers are the most common problems experienced in practice which are yet to be solved (Birks et al., 2006; Cao et al., 2004).
It is now well established that the ability of a bond coat to form α-alumina (one of the chemically most inert and stabile ceramics above 1200 oC) layer with negligible transient oxidation, and the adherence of the alumina to the bond coat (overlay coatings), is a critical factor in controlling the durability of TBCs. The residual stresses in the alumina layer of the bond coat also play an important role in spallation behavior. This stress is defined by the growth stresses and thermal stresses and the stress relaxation which takes place by plastic deformation of the alloy and the oxide; these include the depletion of the scale-forming element, inter-diffusion with the substrate, and deformation association with thermal-expansion mismatch. Therefore, failure of TBCs is generally the result of thermo-mechanical processes accompanied with the crack-formation process (Miller 1984; Stiger et al., 1999; Evans et al., 2001; Kim et al., 2002). For the high temperature applications, ceramics may be a possible solution, but unfortunately they are very brittle and have a little damage tolerance.
The creation of a high temperature metal-oxide composition with the optimal correlation of heat resistance and oxidation resistance, for which the above-mentioned basic requirements will be satisfied, can be realized in accordance to the modern notion on the design of structural materials and could be solved with the innovation strategies of developing graded microstructures materials and internal architecture which permits the consideration of a materials solution optimized for multifunctional requirements (Bouaziz et al., 2008). In recent years, sandwich structures, and more generally hybrid architectural materials, have attracted a lot of interest, caused by the modern technological opportunity of obtaining such materials in the wide range of varying functional parameters.
It is also known that the variety of natural materials is huge and that the secret is the variety of architectures that nature has developed. The natural materials present a number of characteristics very attractive for potential applications – damage tolerance, self assembly, adaptive growth, and self healing characteristics (Vincent 2012; Mann 2001). Self healing materials are recognized as a new class of engineering materials with the capacity to more or less autonomously repair internal damage, and it is clear that the service life of a structure made of such materials will be significantly higher. Therefore, the development of self healing mechanisms for mechanical and thermal activated self healing surfaces made of composite metal-ceramic for mechanical high temperature components (for engines) is a very urgent problem for many technical fields. This design strategy may provide guidelines for future development of structural combined metal-oxide compositional materials by transcribing natural materials (bone, shell, tooth), with superior high temperature mechanical (engineering) performance.
In this context, the results of previous works (Kutelia et al., 2005, 2006a, 2006b, 2007; Tsurtsumia et al., 2005, 2008a, 2008b) dedicated to the investigation of the formation mechanism of Thermally Grown Oxide (TGO) layers on the surface of high chromium content (>40%) Fe-Cr-Al-RE system alloys, has given us the motivation for the application of this alloy as a coating for metals and alloys with the purpose of increasing their high temperature gas corrosion resistance while elaboration of the metal-oxide composition with good correlation of heat resistance and oxidation resistance simultaneously.
On the assumption of the above mentioned, the aim of this work was to show the possibility of the creation of a high temperature structural composition on the basis of refractory metals and alloys with the application of heat resistant coatings of close to equiatomic composition of Fe-Cr-Al-RE (La, Y) system alloys with self-organizing protective TGO.
This research work demonstrates the oxidation resistance of the alloy of Fe-45%Cr-4%Al-0.3%RE (RE=La or Y) is significant for the top coating material on refractory metals (Nb, Mo) and alloys (low alloyed Cr) and develops the basis for new high temperature metal-ceramic hybrid compositions with thermal activated self-healing surfaces.
The substrates were monocrystalline disc specimens of refractory metals such as niobium and molybdenum with the diameter of 15 mm and height of 3.5 mm. These were cut off from the cylindrical boule of corresponding metals, the monocrystals of which were grown with the crystallographic direction <110>. The shear planes are the same as the crystallographic plains (110) with the accuracy not narrower than 10'. The disc shape samples of the substrate had the physical surface with the orientation plans of (110) and  of the corresponding crystals. Shear planes were then bright polished and followed by annealing (in the inert gas atmosphere) at 1500 oC for 60 minutes, and were coated with the heat resistant alloy of Fe-Cr-Al-RE (RE=La or Y) system.
of the corresponding crystals. Shear planes were then bright polished and followed by annealing (in the inert gas atmosphere) at 1500 oC for 60 minutes, and were coated with the heat resistant alloy of Fe-Cr-Al-RE (RE=La or Y) system.
The specimens with the substrate of low alloyed chromium were prepared from cylindrical rods of polycrystalline alloy BX-17-1 (chromium base alloy alloyed with 17% silicon and 1% yttrium, and so labeled as BX-17-1). These specimens were a cylindrical shape of 10 mm diameter and 12 mm in height; their surfaces were polished to a brilliant finish (on grit papers of diminishing size). After coating the surfaces of these specimens with the Fe-Cr-Al-Y system alloy coating, they were cut in two along the longer axes of the sample, for the purpose of investigating the cross-section (the evaluation of coating thickness, investigation of elemental distribution in the depth (into the thickness) of the coating, for the comparative analysis of the morphology and composition of oxides developed on the substrate and coating surface and their interface, as well as in order to evaluate the growth defects in coating and the cracks in it).
The objective of this work was to deposit the oxidation resistant coatings on the surfaces of heat resistant materials. The substrates were chosen based on their composition, and there was applied an oxidation resistant alloy of Fe-45%Cr-4%Al-1%Ni-0.3%RE (RE=La, Y), the high temperature (gas) corrosion resistance of which was investigated in authors' previous papers (Kutelia et al., 2005, 2006a, 2006b, 2007; Tsurtsumia et al., 2005, 2008). The coatings on the chosen substrates surfaces were deposited using the electron-beam physical vapor deposition technique (EB-PVD). The vacuum in the chamber was P0 ~10-5 Hg mm and the deposition occurred by permanent pumping out the chamber. Single-source alloy coating was prepared using cast ingots of the alloys of corresponding compositions of Fe- Cr-Al-Ni-La and Fe-Cr-Al-Y systems that was fed into electron beam and vaporized to deposit on the substrate, in this case, the evaporation rates could be fixed independently to provide the required deposit composition.
On the monocrystalline samples of Nb and Mo, the coatings were deposited out of Fe-45%Cr-4%Al-1%Ni-i 0.3%La alloy while the substrate temperature was Ts =650 oC, which during the coating process was increased (because of the heat transfer of vapor condensation) and at the end of the deposition was ~700 oC (at the given configuration of distance between target and a substrate, and deposition velocity). The coatings of the mentioned composition alloy were formed with up to 10 μm thickness on the surfaces of monocrystalline substrates.
On the surface of polycrystalline samples of BX-17-1 (low alloyed chromium) there were deposited up to 140 μm thick coatings of Fe-45%Cr-4%Al-0,3%Y alloy, with the substrate initial temperature of Ts =700 oC which by the end of deposition process increased up to ~800 oC. The thick coatings had a coarse-grained polycrystalline structure. The physical surface was characterized by surface roughness, the growth defects caused by the final stage of the deposition and presence of intercrystalline (generally) micro-cracks, which propagated up to ~50- 80 μm deep into the deposited coating. The coating thickness of about 30 μm which is adjacent to the substrate-coating interface had a fine-grained structure and was free of growth defects and micro-cracks. In this connection, prior to the chemical-thermal treatment, about ~55 μm thickness layers were ground off and then mechanically polished until developing the Beilby layer17 on the top of the remaining coating layer of ~80 μm thickness.
The thin coatings (~10 μm) deposited on the surfaces of monocrystalline substrates of Nb and Mo, had the physical surface with the same high quality and roughness as the surfaces of the monocrystals and this was caused by their super-fine grain size. Therefore, the surfaces of such specimens did not require mechanical polishing before the thermo-chemical treatment.
All specimens coated with the above mentioned method underwent the cycle of chemical-thermal treatment at 1200 oC and 1400 oC as a result of which there were obtained the compositions with the basis of a heat resistant metallic material (Nb, Mo, low alloyed chromium) and the surface combined protective coating formed on them, which consists of an oxidation resistant sub-layer (out of Fe-Cr-Al-RE system alloy) and a barrier oxide layer above it, which has the composition of Al2O3/(Al2O3 +Cr2O3) and thickness of a few micrometers.
The initial specimens and the samples of synthesized compositions to be investigated by LM, SEM, and WDS methods were shaped to fit into the sample holders of each equipment type.
Figures 1(a) and 1(b) show SEM images taken using Back Scattered Electrons (BSE) from the surface of EB-PVD Fe- 45%Cr-1%Ni-4%Al-0.3%La overlay coating formed on the top of single Nb (110) and Mo (110) crystals, respectively. It is evident that in both cases there are extensive microcracks. Their main initiation factors are individual liquid drops of the evaporated alloy and their solidification on the coating surface, that causes local mechanical (considering high kinetic energy) and thermal (considering fast release of the melting heat) shock in the coating. The existence of such large (tens of microns in diameter) liquid drops in the vapor flow which condenses on the substrate surface is caused by the utilization of direct-flow scheme EB-PVD during the experiments, without special preliminary arrangements against hitting of mentioned drops on the coating surface. Existence of the solidified drops on the coating surface enabled us to make a comparative analysis of the elemental composition of deposited coating and solidified drop and, if needed, corrections in the composition of the ingot-pool of the evaporating target. Thus, in Figures 2(a) and 3(a), there are SEM BSE images of the coated areas with the solidified drops of evaporated alloy on the surfaces of Nb (110) and Mo (110), respectively. It is clear that the drops are of the same diameter (the same mass) when collision with the coating surface on the different materials substrate they solidified with the different velocities; namely, under the solidified drop, on the coating surface of niobium substrate, there is evident four “pancakes” of the sequential solidification that solidified prior to the last, upper semispherical part of the liquid drop (Figure 2(a)), while under the solidified drop on the coating surface of molybdenum substrate, it is observed only one “pancake” (Figure 3(a)), which is a result of different thermal conductivity of the substrates. Therefore, the local pick of the mechanical shock (of the stress) and thermal impact occurred in the moment of drop falling, in the latest case will be much higher, and it is easy to observe as a result the formation of the tracks of microcracks in the coating which are initiated from the solidified drop on the coating surface of molybdenum substrate (see Figure 3(a)). On the assumption of these considerations, it can be concluded that more capable for the crack initiation in the thin coatings of niobium substrate, can be the drops with less diameters (masses), then in the above mentioned case of big drop (when impact point - impulse transfer both mechanically and thermally – is decreased by the formation of a big area of the first “pancake” solidified), but with the same kinetic energy (on the expense of big initial collision velocity).
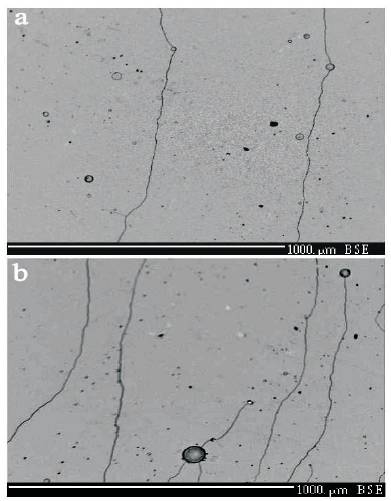
Figure 1. SEM Micrographs showing the Structure of an EB-PVD Fe45Cr4Al1Ni03La Overlay Coating Deposited on the Substrates: a) Single Crystal of Nb, b) Single Crystal of Mo
Figure 2. SEM images and WDS spectra of the EB-PVD Fe45Cr4Al1Ni03La areas deposited at 650 C of single crystal substrate of Nb (110): a) the solidified drop of evaporated alloy splashed on the coating surface, b) higher magnification of the area indicated on (a) which demonstrates the superfine graininess of coating structure, c)WDS spectrum of the solidified drop, d) WDS spectrum of the marked area on the coating
The chosen temperature for the substrate, which was equal to 650 oC, probably was low in order to provide good adhesion of the coating film with the Nb (110) and Mo (110) substrates surfaces, and besides enabled it to reach quite high overheating while condensing on the substrate surfaces. This was the reason for ultra-fine grain structure of both coatings on the substrates and this is demonstrated by the magnified micrographs in the Figures 2(b) and 3(b) of the corresponding indicated areas in Figures 2(a) and 3(a); as it is evident, the coated films of the Fe-Cr-Ni-Al-La alloy (on both substrates while their temperatures was 650 oC) consist of sub-micron grains with very narrow interval of size dispersions.
The comparison of peak intensities of the detected elements (Fe, Cr, Al, Ni) appeared on WDS spectra recorded for the solidified drops (Figures 2(c) and 3(c)) and corresponding marked areas of the coating (Figures 2(d) and 3(d)) unambiguously shows that chemical (elemental) composition of the coating as well as the drops on both substrates, is the same, and coincides with the alloy composition of the following percentage of the components Fe-45%Cr-1%Ni-4%Al-0.3%La (the La concentration is lower than the sensitivity limit of the WDS method for the given element and this is the reason for the absence of its peaks on the spectra). Slightly (~10%) increased intensity of Cr peaks with respect to that of the Fe atoms ones, observed on every spectra of solidified drops (Figures 2c and 3c) and the coating areas (Figures 2d and 3d), is the result of existence of a few mono-atomic thickness chromia layer on the specimen surface, formed at the temperature of ~700 oC (the substrate temperature at the moment of termination the coating process) just after the ending of vapor condensation, owing to interaction of specimen surface with the retained gas in the vacuum chamber the vacuum in which (at the moment of process termination) did not exceed ~5x10-4 Hg.mm.
Figure 3. SEM images and WDS spectra of the EB-PVD Fe45Cr4Al1Ni03La areas deposited at 650 oC of single crystal substrate of Mo (110): a) the solidified drop of evaporated alloy splashed on the coating surface, b) higher magnification of the area indicated on (a) which demonstrates the superfine graininess of coating structure, c) WDS spectrum of the solidified drop, d) WDS spectrum of the marked area on the coating
From the optical microscopic inspection of entire surfaces of Nb (110) and Mo (110) monocrystalline substrates coated by EB-PVD with Fe-45% Cr-1% Ni-4% Al- 0.3% La showed that the coatings surfaces were smooth and with the same high class surfaces as the substrate monocrystals. Besides, on the surface there were not observed the ridges. but the certain solidified drops of evaporated material (alloy). The only defects of the coatings observable by the LM were the single long microcracks the explanation for the origin of which was given above based on the SEM investigations.
Thus, the obtained compositional specimens contained the basis, in the form of heat resistant monocrystalline metals (Nb and Mo), and the coatings on the surfaces of the latest in the form of ultra-fain graininess polycrystalline thin (≤10 μm) film of the corrosion resistant alloy Fe-45%Cr- 1%Ni-4%Al-0.3%La, which has good adhesion with the substrate and is capable to relax in full the strains occurring due to the thermal-expansion mismatch. The result is a material with minimal risk for the development of new microcracks or coating exfoliation while chemicalo thermal treatment of these specimens at 1200 C in order to form the TBC-TGO layer on the coating surface that is analogical to the scale formation on the mechanically polished surface of bulk samples of Fe-45%Cr-1%Ni- 4%Al-0.3%La alloy investigated by the authors previously; (Kutelia et al., 2005, Tsurtsumia et al., 2005, 2008) at that, the authors observed the evolution of already existed (above described) microcracks in the overlay coatings.
Figure 4(a), shows SEM-BSE image of surface area with the microcrack developed during the coating process on the Nb (110) substrate the temperature of which was 650 oC. The image of the same segment after specimen exposure at 1200 oC for 1 hour in air and cooled down to room temperature is presented in the Figure 4(b), where it is evident that together with the surface oxidation of overlay coating there took place the self-healing of existed microcrack.
Higher magnification SEM and LM micrographs of the same area indicated in 4(b) given in Figures 4(c) and 4(d), respectively are showing more details of the microstructure and morphology of healed microcrack and developed “scar” of healed crack. It is clear that both the oxide scale on the coating surface and the coating itself, which healed the microcrack “outfall”, have ultra-fine crystalline structure; and along the discontinuity line track of the micro-crack there is observed the ridge of oxide accelerated growth (Figures 4(c) and 4(d), caused by the increased activity of the material's notched area for the oxidation together with the existence of the nano-stages on these surfaces (the full analogy with the healing morphology of tired skin (tissue) or a bone of the living organism).
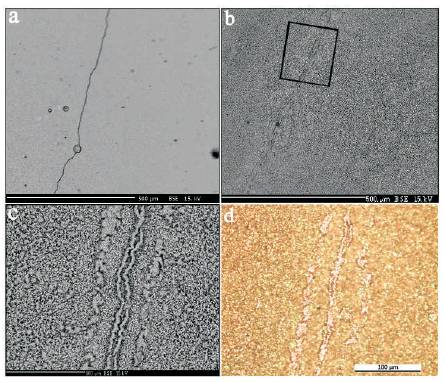
Figure 4. a) Scanning Electron BSE Image of the Area with the Crack in the Thin (≲10 μm) coating of EB PVD Fe-Cr-Ni-Al-La Coated on the Single Crystal of Nb (110), b) Image of the same o area after Specimen Exposure at 1200 C during one hour in air, c) Higher Magnification Micrograph of the area indicated on (b) showing more details of the Microstructure of Healed Microcrack, d) Optical Micrograph of the same Area (c) showing the Scars of Healed Crack
Particularly it should be mentioned that in the given case the morphology, the composition and the thickness of the protective oxide layers on the surface of overlay coatings formed due to the self organizing process at 1200 oC during 1 hour in air, were the same as the ones observed after oxidation of mechanically polished bulk specimens of Fe-45%Cr-1%Ni-4%Al-0.3%La alloy at 1200 oC (Kutelia et al., 2005, 2006; Tsurtsumia et al., 2005).
Figure 5 shows SEM-BSE macrograph and WDS spectra of different areas of EB-PVD of Fe-45%Cr-1%Ni-4%Al-0.3%La coating on the Nb (110) after oxidation at 1200 oC for 1 hour in air. The results of the composition analysis carried out using WDS for the A and B areas marked on Figure 5(a) are presented by the corresponding spectra in Figures 5(b) and 5(c). The oxide film formed on the surface of overlay coating and the film which healed the crack with the outgrowth (ridge) “scar” generally consisted of a mixture of fine-grained Cr2O3 and Al2O3, and a small amount of Fe(Cr, Al)2O4. On the spectra for area B, there is an increased (~20%) intensity for the peak of aluminum atoms (see Figure 5(b)), that is a sign of the fact that during the healing process a main role has the formation of alumina on the discontinuity surface of the crack since a sharp increase in affinity of aluminum atoms towards the oxygen at 1200 oC. Despite the specimens were extracted out of the furnace after the exposure at 1200 oC for 1 hour in air, they were subjected to the cooling down to the room temperature; the SEM and LM methods didnot show the formation of new cracks except of those healed that were existed before the coating oxidation. After repeating the annealing ⇄ cooling cycles (for the same specimens) up to 1200 oC, on the surface of coating + TBC (TGO) composition there were no signs of the cracks as well as slightly noticeable regrowth of the submicron oxide nano-crystallites dimensions were observed in the mixture. The dimensional stability of oxide monocrystals at 1200 oC was determined by the authors previously for the TBC scales formed on the polished surfaces of bulk samples of the Fe-45%Cr-4%Al-0.3%La alloy after its oxidation in air at 1200 oC (Kutelia et al., 2005; Tsurtsumia et al., 2005).
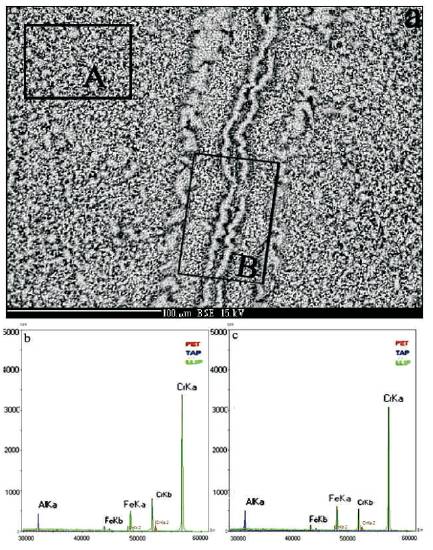
Figure 5. SEM Micrograph and WDS Spectra of EB-PVD Coating on the Surface of Nb (110) Monocrystal showing the Microstructure and Chemical Composition of Self-healed Crack during the Exposure of the Specimen at 1200 oC for one hour in Air, a) BSE Micrograph of the Crack underwent Thermal Activated Self Healing, b) WDS Spectra of the Area A indicated on (a) in a Distance from the Crack, c) WDS Spectrum of area B indicated on (a) of the Healed Crack
Thus, the refractor y metals' monocr ystal base metal/metal composition with an overlay coating of heat resistant alloy of ultra-fine graininess structure, that was synthesized by the authors utilizing the EB-PVD method, after the pre-oxidation at 1200 oC, the mentioned composition transforms into the combined metal/meta/ ceramic high temperature compositional material of the sandwich structure as association the multi-layer architecture of hybrid materials with the distributed different functional high temperature behaviors.
The fine crystalline structure of both the overlay coating and the TGO protective scale on its surface creates the relaxation of the stresses developed due to the thermal-expansion mismatch during the co-deformation of metallic matrix with crystallite layers and providing a smooth transition between the regions with different termophysical and mechanical behavior. Eventually, it will minimize the probability of crack initiation. Each of these three regions of the composition which are burdened the following functions: the basis – providing of the high temperature creep resistance; interlayer of the corrosion resistant alloy – the reservoir of the scale forming elements (Al, Cr, Si, La, Y), which are necessary for the existence of thermally activated self-healing cracks; TGO layer – resistance against high temperature gas corrosion of metal matrix.
The understanding of the structure of different composites requires to study some important effects, such as a damage tolerance, a self-healing capability of cracks, etc.
In Figures 6(a) and 6(b), there are respectively given the optical micrographs of the top surface and a cross-section of EB-PVD Fe-45%Cr-4%Al-0.3%Y thick (135 μm) overlay coating deposited in the above mentioned conditions on low alloyed chromium polycrystalline substrate. It is evident that the superficial part of coating surface has a coarse-grained polycrystalline structure (with the average grain size ~25 μm) and there are long micro-cracks, mainly with an intercrystalline distribution, Figure 6(a). Another characteristic defect of the coating that is clearly defined on cross-section plains were the channels (Figure 6(b)) formed between the columns grown normal along to the substrate on the final stage (i.e. while increased the substrate temperature) of the coating formation which have different lengths from the physical surface down to the ~50-80 μm depth in the coating.
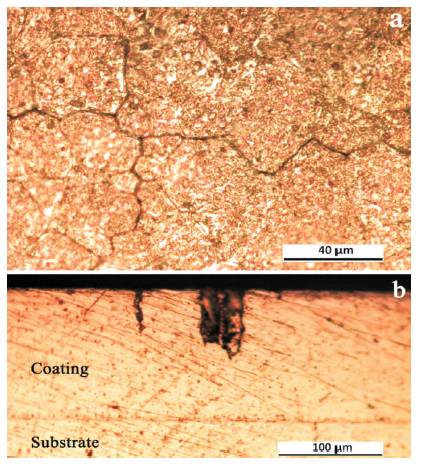
Figure 6. Optical Micrographs showing the (a) Surface and (b) Cross Section of EB-PVD Fe-Cr-Al-Y Overlay Coating Deposited on the Low Alloyed Chromium
Besides, the growing coating layer of the 30 μm, adjacent to the substrate-coating interface is characterized by the fine grained structure (<10 μm) and is a free of defects of above-mentioned type.
Inasmuch as thick coatings physical surfaces have some certain roughness, caused by the final stage of the columnar growth, from the surfaces of such coatings the layers of ~50 μm were ground off and polished until the formation of Beilby layer; thereafter the specimens were exposed in the furnace at a temperature of 1200 oC during one hour and cooled down to room temperature. Figure 7 shows SEM-BSE images of cross-section of an EBPVD Fe-Cr-Al-Y overlay coating deposited on a polycrystalline low alloyed Cr specimen before (a, c) and after (b, d) specimen exposure at 1200 oC during one hour in the ambient of air. On the Figures 7(a), 7(b), 7(c), there is presented the micrograph of polished cross-section plain of the investigated specimen and the corresponding magnified image of the same plain area prior to the specimen thermal treatment. There are evident coating defects in the form of channels of columnar growth and different contrast of the substrate and coating layer in BSE images is also observed. The later is caused by the different chemical composition of the substrate and a coating, which is the reason for sharp visualization of substrate/coating interface. The images of the same areas on the same specimen (and the same magnification) after its exposure at 1200 oC in air and cooling down to the room temperature are given on the Figure 7(b) and 7(d) respectively. There is evident a result of preoxidation where the full healing occurred on the all observable coating growth defects appeared in the forms of channels. The same micrographs evidently demonstrate essential difference between the surface morphology and the structure of the formed scale on the surface of low alloyed chromium (the substrate) and the alloy of Fe-45%Cr-4%Al-0.3%Y (coating); namely, the oxide scale layer on a substrate has a nonuniform uneven (with the ridges) friable structure with the multitude of defects as discontinuity, while the oxide layer on the coating surface has continuous and smooth surface without any buckling and ridges (Figure 7(d)). LM observation of bigger areas of the same specimen before and after high temperature preoxidation, both from the top coating surface of the overlay coating and the cross-section plains, give us reason to conduct the simultaneous comparative analysis of the forming oxide scale developed due to the self-organization process on the surface of coating and a substrate.
Figure 7. SEM–BSE micrographs of the cross-section of EB-PVD Fe-Cr-Al-Y overlay coating deposited at 700 oC on a polycrystalline low alloyed Cr specimen before (a and c) and after (b and d) specimen exposure at 1200 oC for one hour in air, showing the full healing of the coating growth defects in the form of inter-columnar channels (see the text)
Optical micrographs of the top surface overlay coating of the same specimen prior to and after the exposure at 1200 oC for 1 hour in air are illustrated on Figures 8(a) and 8(b), respectively. They evidently demonstrate full self-healing at 1200 oC of the existed micro-cracks on the coating before the exposure. It should be especially remarked that on the continuous oxide scale formed at 1200 oC on the surface of overlay coating, despite very extensive specimen cooling (that is realized by the fast transportation from the heated furnace directly to the room temperature), the formation of micro-cracks was not observed (Figure 8(b) and 7(d)). The original reason for this, may be said, is the fine grained structure of the scale and its self organizing adaptive growth in the initial stage which increases TGO to relax developed stresses. The investigation results of the scale composition, formed simultaneously on the substrate cross-section and an overlay coating, conducted using the WDS microanalysis are shown below. On Figure 9(a), it is shown the SEM-BSE micrograph indicating the composition of the microstructure, which was developed on the crosssectional surface of the low alloyed chromium coated with Fe-Cr-Al-Y alloy by the EB-PVD, the coating after oxidation at 1200 oC during one hour in air. On the shown image, there are marked out three areas for which it was recorded WDS spectra: area A from the coating surface, B – from the substrate/coating interface, and C – from the substrate surface. As it is evident from the spectrum of area A shown on Figures 9(b), 9(c), and 9(d), the scale formed on the overlay coating, which is of fine grained structure is a mixture of each element oxide crystallites containing the overlay coating composition (in the spectrum there are presented the peaks of Cr, Fe, Al, and Y atoms), the most of which is of the chromium oxide (Cr2O3) existed in the mixture. In the area of substrate/coating interface, there are evident the segregations of the inclusions (Y rich particles, which are pretty rough – few times larger than chromium oxide grain sizes) of yttrium oxide (Y2O3) in the interface of scales formed on the substrate surface and a coating (a), that is confirmed by the spectrum peak (c) several times increased in its intensity taken from the area B. The spectrum (d) recorded for the scale on the substrate (area C) shows that the scale formed on the substrate mainly is a chromium (Cr2O3) with the inclusions Yttrium (Y2O3) crystallites in the grain boundaries of rigid structured main oxide in the scale.
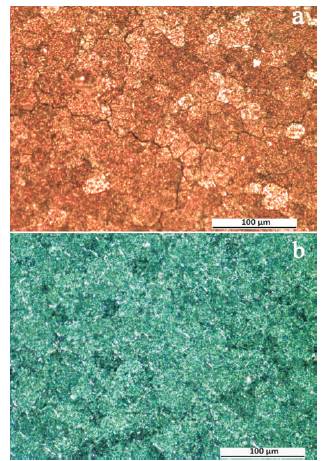
Figure 8. Optical Micrographs showing: a) the Area of Top Surface Coating, the Specimen before High Temperature Exposure, b) the same Area of the Surface after Specimen Exposure at 1200 oC during one hour and Cooling down to Room Temperature
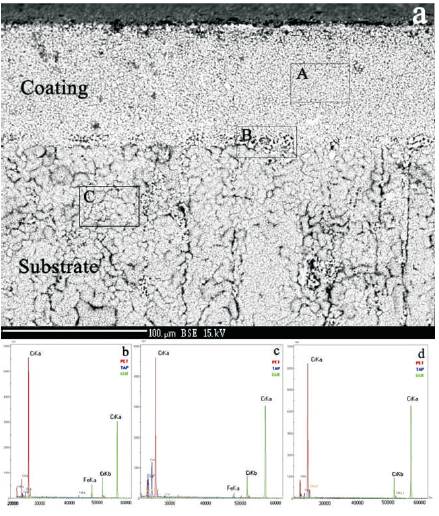
Figure 9. a) SEM-BSE micrograph showing the composition of microstructure that developed on cross-section surface of the low alloyed specimen coated with EB-PVD Fe-Cr-Al-Y alloy. The image after oxidation at 1200 oC during one hour in air; (b-d) WDS spectra of the areas marked on (a) respectively A, B, C
The role of high chromium content in the mechanism producing Al2O3 subscales (in the case of low alloyed chromium and possibly SiO2 subscales) in Fe-Cr-Al-RE alloys can be described by considering the transient oxidation phenomena, determined by the authors for the bulk specimens of Fe-Cr-Al-RE alloy with the high (>40%) chromium content (Kutelia et al., 2005, 2006). The results of later are in a good correlation with the ones observable at the oxidized surfaces of overlay coatings of Fe-45%Cr- 1%Ni-4%Al-0.3%La and Fe-45%Cr-4%Al-0.3%Y alloys. The high chromium content is resulted in the Cr2O3 scale which may be continuous and defines a lower scale-alloy oxygen activity, reduces oxygen diffusion, and curtails internal alumina TGO subscale formation. In Figure 10(a), there is shown SEM image of the specimen surface after oxidation cycles at 1200 oC oxidized totally for 10 hours. On the area with the spall oxide there is displayed two layer levels which are marked as A – the superficial scale layer and B – sub-layer. The magnified image of area B shows that the sub-layer has the nonporous polycrystalline structure with the grain size of ~1 μm (Figure 10 (b)). The registered Auger-spectra for the area A of the scale presented in the Figures 10(b) and 10(c) shows that there are presented all the elements composing the solid solution of metallic matrix, and that all of them are in chemical bounding with oxygen. For aluminum, this appears in the shape of the peak of Al typical of Al2O3 for the Auger-transition KL3L3 and in the shift of energy of this transition to the value 1388 eV. The presence of chromium oxide Cr2O3, together with aluminum oxide is revealed by the typical change of fine structure of Auger-peaks, which are responsible of the oxidation of chromium atoms, namely, intensities of plasmon peaks attenuated, and the intensity of the peak corresponding to Auger-transition L3M23M23 at 489 eV is equalized with intensity of the main peak of L3M23M45 transition at 529 eV. The oxidation of iron atoms and, respectively the presence of the iron oxide in the composition of scale, is revealed by essential widening of Auger-peaks in the triplet of L3VV transition of iron atoms, while the Auger spectrum recorded for the area B is in evidence for that the sub-layer is an alumina (Figure 10 (d)), where two oxygen and aluminum peaks are visible and they are precisely corresponding to that of inherent to Al2O3, at the same energies. This data is in evidence for the main role of transient oxidation phenomenon of the formation TGO-TBC on the surface of Fe-Cr-Al-RE alloy with high chromium content. Eventually, the Al2O3 subscale becomes continuous and rate controlling (Kutelia et al., 2005, 2007, Tsurtsumia et al., 2008). Therefore, by the combination of mentioned factors in the certain temperature range of surface overlay coatings EB-PVD Fe-Cr-Al-RE with the high chromium content alloy, it is possible to create some conditions for the scale development even at early stages, when the scale thickness is several microns. It will create a barrier for the counter-diffusion of cations and anions. This also will create a slow growing layer on the metallic matrix which will be shielding from high temperature corrosion. At the 1200 oC during long time (a few tenths of hours) exposure, the microstructure, the composition and the thickness of the formed scale remains stable without any considerable changes.
Figure 10. a) SEM Image of the Specimen Morphology with Polished Surface after Oxidation at 1200 oC with the Cycles of 1+4+5 hours, b) the Magnified Image of the area B. The Area with Spalled Outer Scale, c) Auger Spectrum of the Area A, d) Auger Spectrum for the Area B
Additional exposure at 1400 oC during 30 minutes of the specimens pre-oxidized at 1200 oC during one hour showed that the scale formed during the pre-oxidation of overlay coating undergoes the refinement of continuity and the structure at the expense of regrowth and a solid phase reactions of submicron crystallite which are composing the scale. And besides there takes place the intensification of reactive element diffusion and their oxides formed in the existed scale micro-channels, which leads to the blocking of short circuit diffusion paths of cations and anions through the scale.
Figures 11 and 12 show the surface morphology after exposure for 30 min at 1400 oC after the initial 1h pre-oxidation at 1200 oC. In Figure 11, it is evident that the scale formed on the overlay coating has a uniform structure but with an enlarged (~5 μm) crystalline oxide and no signs of microcracks in the scale. It is obvious the statistically quite uniform distribution of phase segregations with increased contrast on BSE image (Figures 11(a), (b), and 12(a)). As the local WDS elemental analysis of these segregations showed (Figure 12(b)) comparatively with the areas without the ones (Figure 12(c)), the visible segregations are the Yttrium inclusions which were formed on the surface and an outlet of the easy diffusion micro channels between the re-crystallized chromium oxide grains. Besides, it is evident that around the oxide crystallites boundaries of main components there are presented the aluminum and yttrium ultra dispersive particles as well. As on the spectrum recorded for the area which is free of the abovementioned segregations (Figure 12 (c)), there are presented the peaks of each element correspondent to the coating composition, as well as yttrium peak but reduced 5 times in its intensity.
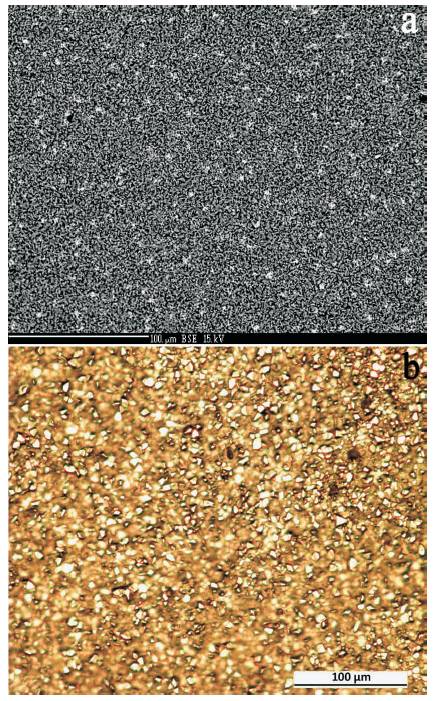
Figure 11. a) SEM-BSE Image and b) Optical Micrograph of the Surface EB-PVD Fe-Cr-Al-Y Coating on the Polycrystalline Substrate of Low Alloyed Chromium after Oxidation Cycles at 1200 oC during one hour and Cooling down to Room Temperature + Exposure at 1400 oC for 30 min in Air and again Cooling Down to Room Temperature
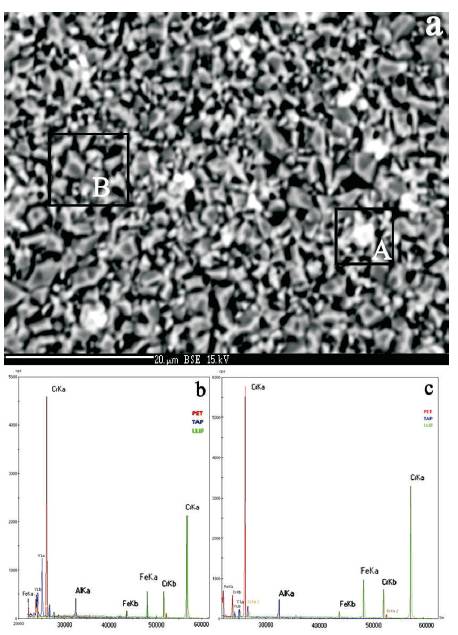
Figure 12. a) SEM-BSE Magnified Image of the Area presented on Figure 11 (a), (b-c) WDS Spectra of the Area marked on a, A and B respectively
In the presented work it is shown that:
The authors wish to acknowledge the financial support received from NASA EPSCoR for this project.