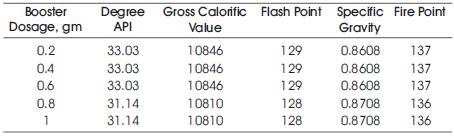
Table 1. Results at a temperature of 60oC for varying Booster Dosages
The new process technologies developed during the past years made it possible to produce biodiesel from recycled edible oils comparable in quality to that of virgin vegetable oil. Biodiesel has an added attractive advantage of being lower in price. Thus, biodiesel produced from recycled edible oils has the same possibilities to be used. From an economic point of view, the production of biodiesel is very feedstock sensitive. From a waste management standpoint, producing biodiesel from used edible oil is environmentally beneficial, since it provides a cleaner way for disposing these products; meanwhile, it can yield valuable cuts in CO2 as well as significant tail-pipe pollution gains. This paper is about the manufacturing of biodiesel from the used vegetable oil. The study aims to define the requirements for biodiesel production by the esterification process, testing its quality by determining some parameters such as Degree API, Gross Calorific Value, Flash point, Specific Gravity and Fire point and comparing it to the commercial Diesel fuel, and the strategic issues to be considered to assess its feasibility, or likelihood of success. The experimentation was carried out for varying booster dosages from 0.2 to 1 gram at 60oC. The experimental results show that the biodiesel obtained at the conditions of oil: alcohol ratio, 6:1, at catalyst dosage 1 gram, at a temperature of 60oC and booster dosages of 0.2 to 1 gram was of good quality.
Bio-energy is obtained from organic matter, either directly from plants or indirectly from industrial, commercial, domestic or agricultural products and waste. The use of bioenergy is generally classified as a carbon-neutral process, because the carbon dioxide released during the generation of energy is balanced and absorbed by plants during their growth [15].
The term bio-energy really covers two areas: bio-fuel which is the transformation of plant materials into liquid fuel, and bio-mass, where solid plant materials are burnt in a power plant and this process creates energy, which can then be for immediate use or stored [12-16].
In the most general sense, biodiesel refers to any diesel fuel substitute derived from renewable biomass. More specifically, biodiesel is defined as an oxygenated, sulfurfree, biodegradable, non-toxic, and eco-friendly alternative to diesel oil. Chemically, it can be defined as a fuel composed of mono-alkyl esters of long chain fatty acids derived from renewable sources, such as vegetable oil, animal fat, and used cooking oil designated as B100, and also it must meet the special requirements such as the ASTM (American Society for Testing and Materials) and the European standards. For these to be considered as viable transportation fuels, they must meet stringent quality standards. Biodiesel is a fuel that can be made from pure or waste vegetable oils such as soya and rape seed (canola) oil, by mixing with methane and a small amount of catalyst. It runs a diesel engine just as petroleum-based diesel would do [8]. In United States, biodiesel is made from soybean oil because more soybean oil is produced in the United States than all other sources of fats and oil combined [1]. Additionally to its environmental characteristics, it is evident, that there is a latent demand of this product because of the recent rises in the price of oil, and the realization that fossil fuels will eventually run out, which resulted in renewed interest in fuel made from plant oils or biodiesel. That is why it is necessary to study the potential of biodiesel, as well as to study its feasibility, if it can be used as a viable alternative fuel in the future [9, 10] . Making biodiesel from waste vegetable oil is one of the most productive ways to utilize waste vegetable oil [7, 11].
The most common way of producing biodiesel is the transesterification of vegetable oils. The methyl ester produced by transesterification of vegetable oil has a high cetane number, low viscosity and improved heating value compared to those of pure vegetable oil which results in shorter ignition delay and longer combustion duration and hence low particulate emissions. Its use results in the minimization of carbon deposits on injector nozzles. The main purpose of the present study is to test its quality by determining the parameters such as Degree API (American Petroleum Institute), Gross Calorific Value, Flash point, Specific Gravity and Fire point and comparing it to the commercial Diesel fuel.
Olutoye and Hameed, 2013, [4]have investigated the catalytic activity of an active heterogeneous Al2O3 modified MgZnO (MgZnAlO) catalyst for the transesterification of different vegetable oils (refined palm oil, cooking palm oil, waste palm kernel oil and coconut oil) with methanol to produce biodiesel. The catalyst was characterized by using X-ray diffraction, Fourier transform Infrared spectra, Thermo gravimetric and differential thermal analysis to ascertain its versatility. Effects of important reaction parameters such as methanol to oil molar ratio, catalyst dosage, reaction temperature and reaction time on oil conversion were examined. Within the range of studied variability, the suitable transesterification conditions (methanol/oil ratio 16:1, catalyst loading 3.32wt%, reaction time 6h, temperature 182oC), the oil conversion of 98% could be achieved with reference to coconut oil in a single stage. The catalyst can be easily recovered and reused for five cycles without significant deactivation.
Thirumarimurugan, et al, 2012 [5]has studied the conversion of waste sunflower oil used for domestic purposes such as cooking oil into biodiesel using an alkali catalyzed trans-esterification process. Reports of experimental data on the production of fatty acid methyl esters from sunflower oil using Sodium Hydroxide (NaOH) as alkaline catalyst were presented. The variables affecting the yield and characteristics of the biodiesel produced from these vegetable oils were also studied.
Padmarag Deshpande and Kavita Kulkarni, 2012, [16] discussed the scarcity of conventional fossil fuels, growing emissions of combustion-generated pollutants, and their increasing costs which make biomass sources more attractive. Biodiesel has become more attractive recently because of its environmental benefits and the fact that it is made from renewable resources. The finite nature of fossil fuels necessitates consideration of alternative fuels from renewable sources. This article reports experimental work on the production of biodiesel by Transesterification from two bio feeds, having less Iodine Value; Palm oil and Ghee (known as Clarified Butter) using alkaline catalyst. The variables affecting the yield and characteristics of the biodiesel produced from these bio feeds were studied. The biodiesel samples were physiochemically characterized according to ASTM standards. From the results, it was clear that the biodiesel fuel produced from Palm oil and Ghee (Clarified Butter) was within the recommended standards of biodiesel fuel and shows promising alternative.
To test the quality of the sample by determining parameters such as Degree API, Gross Calorific Value, Flash point, Specific Gravity and Fire point and comparison of biodiesel with commercial Diesel fuel.
Used palm oil is filtered and water particles are removed to remove the contaminants, then 200ml of this sample is heated to 50-60°C for 10 minutes and then cooled. 0.56 wt % of sodium hydroxide is accurately weighed and mixed slowly with 51 ml of alcohol in a three-necked round bottom flask with continuous stirring. To this 0.23 wt % of booster is added. The mixture is heated at temperatures of 60°C for a time period of 60 minutes at a fixed r.p.m. of 450. After one hour of reaction, the whole system is allowed to cool and it is transferred into a separating funnel and allowed to settle for 12 hours. After proper settling, there will be two layers; one is crude Biodiesel (upper layer) and the other one is crude Glycerin (lower layer). The crude biodiesel is separated from crude glycerin and collected in PET bottles. Then the crude Biodiesel recovered is washed with water to remove wax and alcohol. Two layers are formed, upper layer being Biodiesel and the lower layer being water. Now the Biodiesel is separated from the water by a separating funnel. The amount of Biodiesel recovered and Glycerin are measured and noted.
About 100 mg of each sample was weighed into a 10ml volumetric flask and diluted to the mark with ethanol. 10μL of the resulting solution was injected onto the HPLC (High Performance Liquid Chromatograpgh) column. The amount of individual glycerides present in each sample was determined based on the response obtained from the chromatogram and the calibration function for each glyceride.
Four standards (S1-S4) of oleic acid, monoolein, diolein, and triolein of different concentrations in microgram per millilitre (μg/mL) were used to set up the calibration curve for the determination of free fatty acids which is a function of residual amount of triglyceride and partial glycerides (MG and DG) in biodiesel. The various components in the biodiesel were separated into fractions of Oleic Acid (OA), Triglycerides (TG), Diglycerides (DG) and Monoglycerides (MG) in ascending order of evolution times. The presence of ethanol in the chromatogram is due to the fact the biodiesel samples were diluted in with a hexane/ethanol.
3.2.1 Flash PointThe determination of flash point consists of heating the fuel samples until the minimum vapour is formed to flash on the application of a small flame. Clevland open cup apparatus is used for determining the flash point.
3.2.2 Fire PointThe determination of fire point consists of heating the fuel samples at a standard temperature until the vapour is produced to catch fire, on the application of a small flame. Clevland open cup apparatus is used for determining the fire point.
3.2.3 Kinematic ViscosityKinematic viscosity is determined using Redwood viscometer. Viscometer consists essentially of a cup with an orifice in the center of the base which may be closed by a wall and socket valve, water bath, thermometer, time recorder, etc.
3.2.4 Gross Calorific ValueCalorific Value quantity of heat will include the heat of condensation (latent heat) of the water vapour formed by the combustion of the hydrogen in the fuel, as it cools to ambient conditions.
The experimentation was carried out for varying booster dosages from 0.2 to 1 gms at a temperatures of 60oC. The experimentation results show that the biodiesel obtained at the conditions of oil: alcohol ratio, 6:1, at catalyst dosage 1 gram, at a temperature of 60oC and booster dosages of 0.2 to 1 gram was of good quality as shown in Table 1. Therefore, at the temperature of 60oC, five samples are analyzed using HPLC (High Performance Liquid Chromatography) and the presence of the bound glycerol (MG, DG, TG) is obtained.

Table 1. Results at a temperature of 60oC for varying Booster Dosages
High Performance Liquid Chromatography (HPLC) is another common technique used to analyze biodiesel. It has been used to identify various components of biodiesel mixtures including fatty acid methyl esters, triglycerides, diglycerides, monoglycerides, and fatty acids, among others. HPLC analysis time is generally shorter than GC (Gas Chromatography), and no derivitization step is needed. In an HPLC analysis, the sample is injected into a column with a non-polar stationary phase. A polar mobile phase is then pumped through the column to elute the sample. As the sample moves through the column, the different components begin to separate based on polarity. Retention times for each compound can be recorded and compared to retention times of known standards to identify the different components of the sample. Peak resolution refers to the degree to which one peak is separated from another, and is usually measured by the distance between two peaks at their base. In general measures that tend to increase resolution (such as decreased flow rates) also tend to increase total elution time.
The formation of the biodiesel is confirmed with the presence of bound glycerol (MG, DG, TG) in the product samples. The qualitative and quantitative analysis of the bound glycerol is found using the HPLC analysis. The HPLC analysis of the samples with var ying booster concentrations from 0.2 to 1 gram is shown in Figures 1-5. From the figures, it is observed that DG, MG, TG peaks are obtained at 16, 18, and 7 minutes of retention time. The quantitative data of the total glycerol present in the five samples is given Table 2.
Figure 1. Chromatogram of palm-biodiesel – Sample 1, Wt % of Oil: Methanol: Catalyst: Booster = 6 : 1 : 0.56 : 0.11
Figure 2. Chromatogram of palm-biodiesel- Sample 2, Wt % of Oil : Methanol : Catalyst : Booster = 6 : 1 : 0.56 : 0.23
Figure 3. Chromatogram of palm-biodiesel – Sample 3, Wt % of Oil : Methanol : Catalyst : Booster = 6 : 1 : 0.56 : 0.34
Figure 4. Chromatogram of palm-biodiesel – Sample 4, Wt % of Oil : Methanol : Catalyst : Booster = 6 : 1 : 0.56 : 0.45
Figure 5. Chromatogram of palm-biodiesel –Sample5, Wt % of Oil : Methanol : Catalyst : Booster = 6 : 1 : 0.56 : 0.57
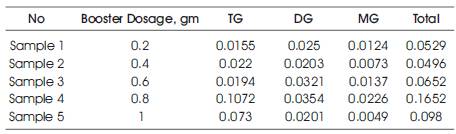
Table 2. Compositions of individual glycerides present in the five samples that are analysed using HPLC
The physic chemical parameters like its Density, Gross Calorific Value, Specific gravity, Kinematic viscosity, Color, Volatility characteristics, Flash Point and Fire point were determined.
4.2.1 Effect of temperature on booster dosage for Flash PointThe effect of temperature on booster dosage for flash point was studied at the conditions of oil: alcohol ratio, 6:1, at catalyst dosage 1 gram, at a temperature of 60oC and booster dosages of 0.2 to 1 gram. It was observed that with the decrease in the booster dosage the temperature increased the flash point. Thus, it was observed that the equilibrium conversions increased substantially for every degree rise in temperature. Hence, rate of reaction is strongly dependent on temperature of reaction [2]. Therefore the optimum value of Flash Point was obtained at 0.8 gram of the booster dosage as shown in Figure 6.
4.2.2 Effect of temperature on booster dosage for Fire PointFlash point and fire point are important temperatures specified for safety during transport, storage and handling. From Figure 7, it is concluded that, with the decrease in the booster dosage the temperature increased the fire point. Therefore the optimum value of Fire Point was obtained at 0.8 gram of the booster dosage. Fire point decreases after transesterification, which shows that its volatile characteristics had improved and that it is also safe to handle.
4.2.3 Effect of Gross Calorific Value on booster dosageFrom the Figure 8 it is concluded that, with the decrease in the booster dosage, the Gross Calorific Value increased. Therefore the optimum value of Gross Calorific Value was obtained at 0.8 gram of the booster dosage.
4.2.4 Effect of Degree API on booster dosageFrom the Figure 9 it is concluded that with the decrease in the booster dosage, the Degree API value increased. Therefore the optimum value of Degree API was obtained at 0.8 gram of the booster dosage.
4.2.5 Effect of Specific gravity on booster dosageLower value of the specific gravity of the final product is an indication of completion of reaction and removal of heavy glycerine. The influence of molar ratio, temperature and catalyst quantity on the specific gravity of the biodiesel was studied by Miao and Wu [3], 2006. From Figure 10, it is concluded that with the increase of the booster dosage the specific gravity increased. The optimum value of Specific gravity was obtained at 0.8 gram of booster dosage.
This study is about the manufacturing of biodiesel from waste vegetable oil. This study showed that biodiesel is environment friendly, due to its less polluting and renewable nature compared with conventional petroleum diesel fuel. Moreover, it could be used in any diesel engine without modification. Biodiesel could be made out of pure or waste vegetable through a transesterification process in the presence of a catalyst and a booster. The purpose of the transesterification process is to lower the viscosity of the oil, which is better for the engine performance. Biodiesel fuels produce slightly lower power and engines consume more fuel than diesel fuel. The cost of biodiesel varies depending on the base stock, geographic area, variability in crop production from season to season, the price of crude petroleum, and other factors. The high price of biodiesel is by large due to the high price of the feedstock. However, biodiesel can be made from other feed stocks of low cost oils and fats such as restaurant waste and animal fats that could be converted into biodiesel. The problem with processing these low-cost oils and fats is that they often contain large amounts of Free Fatty Acids (FFA) that cannot be converted into biodiesel using an alkaline catalyst. If biodiesel could be used successfully, it would be beneficial to the environment.
The experimentation results show that the biodiesel obtained at the conditions of oil: alcohol ratio, 6:1, at catalyst dosage 1 gram, at a temperature of 60ᵒC and booster dosages of 0.2 to 1 gram was of good quality. It was observed that adding the booster resulted in less soap formation.