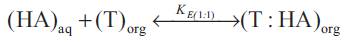
Temperature effect is an important subject of study in the reactive extraction process in view of operating temperature and back extraction/regeneration step. Usually in industrial scale, for production of carboxylic acids, fermentor operates in the temperature range of 303 to 313 K. Thus, an extractant can be considered as good, only if it could operate efficiently in this operating range. The study, thus aims two objectives. (1) To find the effect of temperature on extraction of the propionic acid (2) Whether temperature swing extraction/regeneration can be employed for recovery of the acid. Three types of extractants: tri-n-butylphosphate, tri-n-octylamine and Aliquat 336 were used in different diluents and the thermodynamics of extraction process was discussed in terms of enthalpy and entropy of reaction. Extraction of propionic acid using TOA in different diluents resulted in negative values of enthalpy and entropy. Thus, the reactive extraction process is exothermic in nature and decrease in entropy is obtained. Aliquat 336 in 2-octanol shows the similar behavior as TOA in different diluents but the entropy and enthalpy values were lower, thus by suggesting not so high exothermic nature of the extraction process and not so strong acid Aliquat 336 complexation as acid TOA complexation. Two departures in the normal trend were observed when Aliquat 336 was used in oleyl alcohol and TBP was used in MIBK. In both cases an increase in KE was observed upto a certain temperature and whereupon a decrease was observed. The effect of temperature was correlated in terms of the heat of transfer from organic to aqueous phase (ΔH transfer). It was found that the higher the ΔH transfer for a particular diluent, higher is the temperature effect.
Industrial scale fermentors for production of carboxylic acids operate in the temperature range of 305 to 313 K. Thus, reactive extraction process for recovery of these acids requires an extractant that could operate efficiently in this operating range. Usually, extraction is an exothermic process and a decrease in extraction is expected as the temperature is increased. However, it can be stated that the decrease in extraction is the function of the extractant and diluent chosen i.e. the extracting medium. Thus, it makes the study of temperature effect important when the process is to be designed. Further, the variation in climate, feed and bioreactor conditions make the study of temperature effect more important. The study thus aims two objectives, (i) To find the effect of temperature on extraction of the acid from fermentation broth (ii) Whether temperature swing extraction/regeneration can be employed for recovery of the acid.
Baniel et al. [1,2] studied the effect of temperature on extraction of citric acid using tridecylamine in petroleum fractions with alcohol as modifier, in xylene and in nitrobenzene and observed a sharp decrease of distribution of acid with temperature. Similar results were obtained by Wennersten [3] for the extraction of citric acid by Alamine 336 in a variety of diluents at 25 and 60oC Where, the distribution ratios decreased by as much as a factor of 6. For the extraction of lactic, tartaric, succinic, and citric acids by trilaurylamine in xylene at 20, 30,40, and 50o C, for the 30o C temperature increase, distribution ratios decreased by factors between 2 and 10, depending upon the type of acid [4].
King and Tamada [5] studied the effect of temperature on extraction of succinic and lactic acids by Alamine 336 in MIBK (0 - 75o C) and chloroform (0 - 55o C). Extraction decreases with the increase in temperature. The apparent enthalpies of association are more exothermic for succinic acid than for lactic acid and more exothermic in chloroform than in MIBK. The apparent entropy decrease is greater for succinic acid than for lactic acid and greater in chloroform than in MIBK. For the systems studied, (1:1) complexation is much more exothermic and involves a much greater loss of entropy than the formation of (2:l) or (3:l) complexes. This is reasonable when related to the findings of author in earlier article [6] in which it was concluded that (1:l) complexation involves the formation of an ion pair, but higher complexes involve hydrogen-bond formation. The relatively large differences in enthalpy and entropy loss between the two diluents for (1:l) complexation are consistent with the conclusion that interaction of chloroform with the complex is specific hydrogen bonding. The association of chloroform with the complex is exothermic and increases the order (decreases the entropy) of the system.
Harington and Hossain [7] studied the effect of temperature (10 to 40o C) on extraction of lactic acid using 20% TOA in sunflower oil at natural pH. Increasing the operating temperature increased the distribution coefficient of acid in the organic phase. The trend was suggested to be a positive result since the optimal temperature of fermentation broths is around 38°C. So performing the extraction at this temperature would allow greater extraction than at room temperature. Keshav et al. [8] studied reactive extraction of acrylic, propionic and butyric acid using Aliquat 336 in oleyl alcohol to study the effect of temperature (305 K to 333 K). Effect of temperature on partition (P) and dimerization (D) coefficient was evaluated and it was found that P decreases with increase in temperature whereas varying results was obtained for effect of temperature on D. Chemical extractions using Aliquat 336 in oleyl alcohol at temperatures from 305 K to 333 K shows increase in KE(1:1) values with temperature upto 313 K for acrylic and propionic acid whereas for butyric acid it decreases with increase in temperature over the range studied. Difference in hydrophobicity value and octanol water partition coefficient was suggested as the reason for difference in extraction by the respective acids using Aliquat 336 in oleyl alcohol. Enthalpy (ΔH) and entropy (ΔS) of reaction were evaluated at different temperature and their difference was described in view of different parameters.
The above literature signifies that study of effect of temperature is important in operation and design of a reactive extraction process. A lot of work on reactive extraction of carboxylic acids using different extractants and diluents [9 -25] by the authors could be found in literature. Very little work on the effect of temperature on the extraction of different carboxylic acids is available and particularly for propionic acid. Thus, with this aim, effect of temperature on reactive extraction of propionic acid was studied using different extractants and diluents. In the category of extractants, TOA, Aliquat 336 and TBP were employed. These extractants were used in different diluents. Choice of diluents was particularly in terms of its high solvation ability (degree of extraction) and better solvation medium (the one that could result in no problems of three phase formation). All the selected diluents were found to be efficient in extraction of the acid from dilute solutions. Thermodynamics of extraction was also discussed in terms of enthalpy and entropy of process. The nature of extraction process by different extractants in different diluents was used to evaluate its applicability for applying it to an actual industrial scale process.
TBP (Himedia, India), a phosphorous bonded oxygen donor, a light colorless liquid with the molecular weight of 266.32 g/mol and density of 0.92 g/cm3 ; Aliquat 336 (Methyltricaprylammonium chloride), a quaternary amine with molecular weight of 404.17 and density of 0.888 g/cm3 and TOA (C24 H51 N) (ACROS, India), a tertiary amine, a light colorless liquid with the molecular weight of 353.66 and density of 0.809 g/cm3 were used as extractants. Propionic acid (99%) (Himedia, India) and diluents methyl isobutyl ketone (MIBK), 1-decanol, 2- octanol, oleyl alcohol are of technical grade and were used without pretreatment. Distilled water was used to prepare the solutions of various concentrations of propionic acid solutions. NaOH used for the titration is of analytical grade and was supplied by Ranbaxy, India. For the standardization of the NaOH, oxalic acid (99.8%) was obtained from S. d. fine-Chem Ltd, India. Phenolphthalein solution (pH range 8.2-10.0) was used as an indicator for titration and was obtained from Ranbaxy, India. The range of pH for the experiment was 2.65-3.14 at 303 K. Low concentration of propionic (0.05 0.4 kmol/m3 ) acid was used because in waste streams and in fermentation broths, its concentration is not expected to be greater than 0.5 kmol/m3 [26].
The extraction experiments were performed using a temperature controlled water bath shaker at room temperature. Equal volumes (25 cm3 ) of aqueous and organic phases were shaken for 12 hours and then left to settle for at least 2 hours [6] at a fixed temperature and atmospheric pressure (101.2 kPa). The temperature was then varied from 313 K to 333 K and the procedure repeated to study the influence of temperature. Aqueous phase acid concentration was determined by titration with NaOH. Fresh NaOH was prepared every time prior to titration. Few experiments were carried out in duplicate and consistency was found within ± 2 %.The acid content in the organic phase was determined with a mass balance.
The reactive liquid-liquid extraction of propionic acid ([HA]) with extractant ([T]) gives reaction complex (T :( HA)n ) which remains largely in organic phase. Keeping in view that the exact chemistry involved in the uptake of extra acid is unknown, the distribution coefficient can be interpreted by the following set of equations,
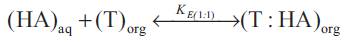
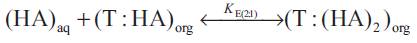
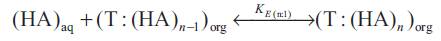
where the subscripts 'aq' and 'org' refers to aqueous and organic phases and n is the number of acid molecules complexes with extractant. The equilibrium complexation constant (K E(n:1) for the reaction represented by above equations as,
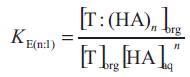
(K E(n:1) was expected to depend on properties of the acid and the solvation efficiency of diluent used. The extent to which the organic phase can be loaded with carboxylic acid is expressed as the loading ratio, z;
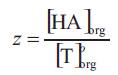
Superscript 'o' represent the initial concentration value. The value of z in equation (5) depends on the extractability of the acid (strength of the acid-base interaction) and its aqueous concentration. The stoichiometry of the overall extraction reaction depends on the loading ratio in the organic phase, z. Since the extraction of propionic acid using different extractants resulted in loading ratios less than 0.5 [[8], [14-22]], only (1:1) acid extractant complexes are formed and thus and (K E(n:1) can be replaced by KE i.e. the equilibrium complexation constant for (1:1) acid extractant complexation and the following equation holds,
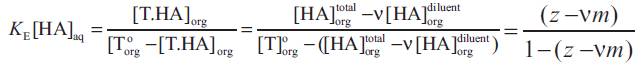
Where 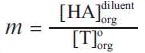 , ν in the volume fraction of diluent and
, ν in the volume fraction of diluent and 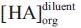 concentration of acid in the organic phase when extracted by diluent alone.
concentration of acid in the organic phase when extracted by diluent alone.
Table 1 reported the chemical equilibria and KE values for extraction of propionic acid using TOA in 1-decanol, 2- octanol and ethyl acetate respectively.
Table 1. Effect of temperature on the reactive extraction of propionic acid (0.05 0.4 kmol/m3 ) using different extractants (TOA, Aliquat 336 and TBP) in volume fraction of 40% in different diluents.
If the enthalpy and entropy of reaction are assumed to be constant over the temperature range [5], the equilibrium complexation constant is related to temperature as:
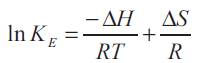
The above expression indicates that a plot of In KE vs 1/ T gives a straight line. The more exothermic the reaction, the more sensitive the equilibrium is to changes in the temperature. The slope is proportional to the enthalpy of reaction, and the intercept is proportional to the entropy. The values of enthalpy of reaction (ΔH), and entropy of reaction (ΔS) were obtained and Tabulated in Table 2. Negative value of ΔH suggests that the reaction extraction of propionic acid using TOA in the different diluents and Aliquat 336 in 2-octanol for T = 305 to 333 K and Aliquat 336 in oleyl alcohol and TBP in MIBK for T = 313 to 333 K, is exothermic in nature in these temperature ranges. Aliquat 336 in oleyl alcohol and TBP in MIBK shows a slight increase in KE values as temperature was varied from 305 to 313 K, E thus in this temperature range endothermic behavior was observed. Further, decrease in entropy may be due to increase in order of system owing to complex formation and due to lowering of degree of freedom (degree of freedom decreases as two compounds combined to form one)
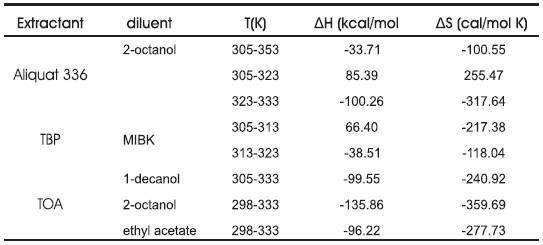
Table 2. Summary of thermodynamics of extraction of propionic acid using different extractants in different diluents.
Extraction of propionic acid by extractant is via intermolecular hydrogen bonding or ion exchange of the extractant group with the acid. The extraction of propionic acid by extractant - acid complexation is expected to be exothermic and makes the system more ordered. Table 2 summarizes the thermodynamics of propionic acid extraction using different extractants and diluents. Extraction of propionic acid using TOA resulted in negative values of enthalpy and entropy. Thus the reactive extraction process is exothermic in nature and decrease in entropy is obtained. Higher the negative value of ΔH, more exothermic is the extraction. The order of exothermic nature of reactive extraction process using TOA as extractant is as: 2-octanol > 1-decanol > MIBK. Similar trend is with ΔS. TOA in 2-octanol gives the highest negative values of ΔH and ΔS among all the categories discussed.
The decrease in entropy may not be accepted from the definition of second law of thermodynamics because all spontaneous processes proceed in the direction of maximum probability, i.e. greatest randomness. However entropy may be looked upon as measure of randomness which is a minimum in systems with an orderly arrangement. For example, take the case of spontaneous conduction of heat along a metallic bar. This results in a random distribution of kinetic energy of the molecules. Rejection of heat from system decreases the disorder of the molecules, an equivalent or greater amount of disorder results in environment. The combination of two molecules into one i.e. the complex resulted in decrease in the degree of freedom and thus a more ordered arrangement. Thus decrease in entropy is expected.
Further a more decrease in order of a particular interaction suggests the stronger interaction of acid with the extractant. The decrease in extraction with temperature suggests that a temperature control should be provided whenever these extractants were used in - situ with the fermentation broth as temperature increase could severely affect the process economics. On the other hand an advantage can be extracted out of this behavior on account of regeneration of the acid from loaded organic phase by temperature swing regeneration.
Aliquat 336 in 2-octanol shows the similar behavior as TOA in different diluents but the entropy and enthalpy values were lower, thus suggesting not so high exothermic nature of the extraction process and not so strong acid Aliquat 336 complexation as acid TOA complexation. Two departures in the normal trend were observed when Aliquat 336 was used in oleyl alcohol and TBP was used in MIBK. In both these cases an increase in KE was observed upto a certain temperature and whereupon a decrease as expected was observed. This can be considered as a positive sign since most fermentation broths operates between 305 to 313 K and optimum performance of an extractant in this temperature range would be a advantageous point of that extractant. Another point that can be discussed is that in both these cases, throughout the studied temperature range, there is not a high decrease or increase in both KD and KE values as were found in extractions involving TOA. Though on one side this is an advantage as these extractants provides a uniform performance in the studied temperature range, yet on the other hand this closes the door for regeneration of the acid by temperature swing regeneration, which was another objective of the present work.
Effect of temperature is attributed to the effect of different parameters like the acid pKa, the extractant-acid interaction, the solubility of the acid in both phases, the extractant basicity and water co-extraction. These parameters were studied by Canari and Eyal [27]. They concluded that the pKa values of common carboxylic acids decrease only slightly when the temperature is increased. Similarly, pKa measurements for carboxylic acids exhibit a small decrease when the temperature is increased but only up to a given point. Above it, the pKa value increases. Similarly solubility of acids is affected by temperature in both the aqueous and extractant phase. The solubility of oxalic, malonic, succinic, adipic, maleic, malic, citric, and tartaric acids in water increases when the temperature is increased from 278.15 K to 338.15 K [28]. Higher the -ve value of ΔH for a particular acid suggests that more exothermic the extraction. This may be accounted due to the difference in partial molar heat of mixing of the complex in the solvent and the partial molar heat of mixing of the acid in the aqueous phase, or ΔHtrue. King et al. [6] evaluated organic phase heat of mixing using partition coefficient values (P) for extraction of acid by diluents alone using the following relation:
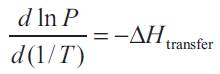
where ΔHtransfer is the heat of transfer from organic to aqueous phase. Attempts have been made to correlate the temperature effect to the (ΔHtransfer).
Figure 1 shows the values of P evaluated for ethyl acetate, 1-decanol and 2-octanol at different temperatures. The data was fitted to equation (8) and the slope provides the (ΔHtransfer ) values as: 1625, 742.01 and -609.7 kcal/mol for ethyl acetate, 1-decanol and 2-octanol respectively. Thus higher is the ΔHtransfer value for a particular diluent; higher is the effect of temperature as can be seen when these diluents were employed with TOA for extraction of propionic acid.
Effect of temperature on reactive extraction of propionic acid was studied. In the extraction of propionic acid using TOA in different diluents (2-octanol, 1-decanol and ethyl acetate) and Aliquat 336 in 2-octanol, negative values of enthalpy and entropy were obtained for the studied temperature range (305 to 333 K). Thus the reactive extraction process is exothermic in nature and increase in order of system was observed. The higher exothermic nature of extraction process and greater increase on entropy was obtained for TOA than Aliquat 336 for the same diluent (2-octanol), thus signifying that the complexation of acid is stronger with the former The lowering of extraction with temperature suggested that during extraction it is desired to maintain the temperature close to 305K so as to get higher results. Temperature swing method of regeneration of acid can be employed for the recovery of acid from these extractants as increase in temperature lower the extraction extensively. On the other hand in systems Aliquat 336 in oleyl alcohol and TBP in MIBK extraction first increases and them decreases as the temperature was varied in the range 305 to 333 K. Thus on one hand these extractants can be successfully employed for extraction from fermentation broths without any temperature control, yet they cannot be recovered by temperature swing regeneration technique. Thus it can be suggested that the complete thermodynamic study is desired for the complete reactive extraction design for recovery of acid from fermentation broth because similar extractant can behave differently in different diluents.
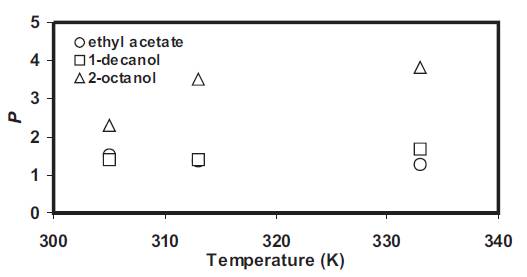
Figure 1. Effect of temperature (305 333 K) on the partition coefficient of different diluents.
Department of Science and Technology (DST), INDIA, for financial support under Young Scientist Project, SR/FTP/ETA- 43/2005, Reactive Extraction of Propionic Acid (PI: Dr. Kailas L. Wasewar, VNIT Nagpur, India).