This paper investigates the oil potential of chestnut samples. Proximate analysis was conducted on the extracted oil to determine its physico–chemical properties. Results showed maximum oil yield of 41.9 % at 55⁰C and lowest values of 37.0 % at 65⁰C, highest and lowest acid content of 25.52 mg/KOH/g at 60⁰C and lowest value at 5.89 mg/KOH/g at 50⁰C. The Free Fatty Acid (FFA) content obtained were 12.76 at 60⁰C and 2.94 at 50⁰C, while Saponification values were 61.71 mg/KOH/g at 60⁰C and 32.25 at 50⁰C. The highest Peroxide value of 92 was recorded at 55⁰C which dropped to 43.4 at 65⁰C. The highest Refractive Index value of 1.463 was obtained during sun-drying treatment while the lowest value of 1.460 was recorded at 65⁰C. Chestnut possess a high level of commercial value that could meet domestic and industrial oil demands.
Agricultural products are classified into oil-seeds (cotton, castor, sunflower, etc), nuts (coconut, groundnut, shea nut, etc) and mesocarps or fruits (oil palm). Plants bearing these agricultural products have greatly contributed to the economic development of many countries where the products are grown for commercial purposes.
Of the many types of oil that can be obtained from these products, only few are very significant in terms of world production and traded as major commodities (Ibrahim and Onwualu 2005). However, the oil extracted from these products has diverse domestic and industrial uses. The oil serves as a major source of vegetable oil that constitutes a good percentage of meal in the diets of common people. The oil as well as the by-products are also very useful as food and non-food materials for the production of snacks, cake, margarine, biscuit, cosmetics, detergent, plastics, etc (Ibrahim and Onwualu 2005).
In most of the developing countries, there has been a steady growth in the demand of edible oil both for domestic and industrial uses. Samuel and Alabi (2012) noted that, vegetable oil productivity in India is too poor to meet the demands. They further stated that, estimates of the total money India spends to import vegetable oil from Malaysia in 2001 alone was 186.65 million U. S. Dollars.
It has been estimated that production-consumption margin is increasing by thousand tonnes naturally because of population growth and rise in per capita income. This situation requires a serious consideration for effective futuristic planning of maximizing oilseed production in the country (Samuel and Alabi 2012). Undoubtedly, such a measure is the crying demand of the country from the agricultural sector. Samuel (2006)reported that, the government has for the first time, recognized the need to reduce the dependence on import of edible oil, and to develop and sustain price stabilization mechanism for oilseeds in the country. India should also borrow a leaf from this and develop the oil sources for sustainability by increasing productivity of the industry. Good quality edible oil is fresh, free from odour and any trace of rancidity. The acceptability of the products at world edible oil market depends on its ability to satisfy basic standard tests for fats and oil (Takakura 2008).
India is a country with generous land; wide varieties of soil types throughout the different agro-ecological zones and climate ranging from tropical to temperate, which makes it possible to grow assorted oilseeds from which vegetable oils can be processed. Groundnut and oil palm are the two major oil crops grown in India and most other Asian countries (Ibrahim and Onwualu 2005).
The labor and high capital involved in the production has perhaps reduced the number of farmers going into production of these two crops in India. India is declining in groundnut production due to the petroleum product boom of 1970. Also the intensive labor involved in groundnut planting and timeliness in harvesting has discouraged most farmers from going into groundnut production.
Therefore there is a need to find alternative source of vegetable oil to complement the existing oil sources in order to meet both domestic and industrial demands. Such crops that are perennial in nature, (to reduce the problem of re-planting every season like groundnut) which can be harvested easily without posing danger of injury; need to be considered in earnestly. One of such crops is chestnut trees.
Chestnut is one of the most important forest species in Southern Europe. Besides, for the intrinsic interest in the ecological aspect, this crop has been widely cultivated for the production of timber and fruit. It has been an important food source for the rural areas of mountain forest and hills; chestnuts are used mainly for the production of flour. Chestnuts are formed in pods which split naturally after maturity to discharge the nuts. The seed nut is further processed by removing its outer cover mostly using manual methods.
Chestnut is planted in India as ornamental plants at homes and the seed nut is often wasted away. Chestnut could be cultivated over a large area of land. There is thus a great potential of production of chestnut and invariably its oils. Chestnuts are grown into some species called chinquapin. It is a genus of eight and nine species of deciduous trees and shrubs in beech family. However, there is need to extract oil from the seeds of chestnut, because this will assist in augmenting the shortfall in production caused by already existing oil.
The objective of this work is to investigate the potential of oil from chestnut grown in India and the possibility of recommending it as substitute or complement for common existing vegetable oils.
The seeds used for the study were obtained from the Girijan Cooperative Society in Andhra Pradesh. The fresh nuts (Plate 1) from the chestnuts plant (Plate 2) were handpicked from the trees and some were obtained by breaking the matured pod (Plate 3) which is shown in Figure 1.

Figure 1. Material Preparation (Plate 1-4)
They were initially sundried, because most of the seeds were still fresh. After sun drying, the nuts were sorted out by removing decayed/ defective and immature seeds to ensure the quality of extracted oil. 1000g of the sorted chestnut was divided into five parts of 200g each. These were later manually shelled to obtain the kernel.
One of the portions of the shelled kernel was sun dried for five hours, while the other four parts were oven dried at different temperatures of 50, 55, 60 and 65oC for five hours. The five samples were then milled to powder form using electric blender to allow easy oil extraction (Adebayo, S.E., Orhevba, B.A., Adeoye, P.A., Musa, J.J. & Fase, O.J. 2012). Solvent extraction method was used to extract oil from milled chestnut using soxhlet extractor (Avalier model) (Plate 4).
100 g of the milled sample was wrapped with filter paper. This was inserted into the condenser of the extractor. The round bottom flask was filled with the solvent (n-hexane) up-to two-third capacity of the flask. The reflux condenser was filled to the top of the extractor and water flow was turned on.
The round bottom flask was placed in the heating mantle and temperature of the mantle adjusted to 1500C so that the solvent is brought to the evaporation point. Each extraction occurred over a period of 8 hours. When the solvent had just siphoned over the barrel, the condenser was detached and the thimble removed. The filtrate was kept in desiccators and allowed to cool at room temperature. The residue solvent was allowed to evaporate.
These procedures were repeated for each of the five samples (sundried sample, 50, 55, 60 and 65oC). The extracted oil was re-heated to remove the n-hexane from the oil through evaporation.
While evaluating the oil potential of the extracted oil, the Refractive index, Density, Saponification value, Acid value, Free Fatty Acid (FFA) Specific gravity, Moisture content, Peroxide value, Freezing point, Melting point, and Oil yield were determined using Standard method given by AOAC. AOAC (2012).
Refractive index is also known as index of refraction, is a measure of the blending of a ray of light when passing from one medium into another. It can be also defined as a dimensionless number that describes how light or any other radiation propagates through the medium. In this research work, the refractive index was determined using the refractometer (Erma hand refractometer) as used by (Ayo and Agu 2012). It has been in the range of 0- 32%. A drop of the oil was placed on the surface of the refractometer and the reading was taken.
The saponification value of an oil or fat is the number of milligrams of potassium hydroxide required to neutralize the fatty acid resulting from the complete hydrolysis of 1g of the sample (Ayo and Agu 2012). In determining the saponification value of the acid, 2g of the oil fat was weighed into a flask, 25ml pipette containing alcoholic potassium hydroxide solution was poured into the conical flask containing the oil, and it was attached to reflux condenser and was heated on boiling water bath for 1 hour with occasional shaking, after which 1ml of the phenolphthalein solution was added and was titrated while hot with standard hydrochloric acid. This procedure was repeated for each of the five samples. Then the blank (that is potassium hydroxide solution without oil) was titrated using the same procedure. The sapnification value was then determined using the relation in equation (1).
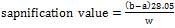
where; a = titrate of the sample, b = titrate of the blank and w = weight of sample.
The acid value of an oil fat is the number of milligrams of potassium hydroxide required to neutralize the free acids resulting from the complete hydrolysis of 1g of the sample (Ayo and Agu 2012). In determining the acid value, 25ml diethyl ether with 25 ml ethanol was mixed and 1.0ml of 1.0% phenolphthalein solution was neutralized and was titrated with 0.1 ml sodium hydroxide solution and then 1- 10g of the oil in the neutralized solvent mixture was dissolved and was titrated with 0.1ml sodium hydroxide solution. The acid value was therefore determined using equation (2).
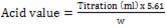
The concentration of peroxides in oil gives an indication of the extent of spoilage. The oil was treated with potassium iodide in an organic solvent. The peroxide liberates the iodine from potassium iodide. The iodine was titrated with standard thiosulphate (Ayo and Agu 2012).
In determining the peroxide value of the acid, the test was carried out in subdued daylight and 1g of oil was weighed into a clean dry boiling tube, 1g of powdered potassium iodide and 20ml solvent mixture was added to the oil that was inside the boiling tube then, it was placed in a boiling water bat for about 60seconds. After that the contents of water was poured into it and the washing was added to the titration flask, containing 20ml potassium iodide solution. The tube to be used was washed twice with 25 ml portion of water and the washings were added to the titration flask. Finally it was titrated with 0.002ml. thiosulphate, using starch as indicator. The peroxides value was therefore determined using equation (3).
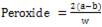
where; a = titrate value for the sample, b = titrate value for the blank and w = weight of sample.
Moisture content is the quantity of water contained in a material. This is based on loss of weight on drying at an oven temperature of 105oC. Besides water, the loss will include the other matter volatile at 105oC (Ayo and Agu 2012).
In determining the moisture content of an acid, a clean flat dish of silica, platinum was dried. The cooled dish was weighed (W1) and 5g of the oil was introduced into the dish and was spread after which it was weighed accurately (W2). The dish and its content W2 is transferred into an air oven at 105oC to dry for about 3 hours. Then a pair of tongs was used to transfer the dish into desiccators, allowing it to cool down in the oven for half an hour and was allowed to cool in the desiccators after which it was then weighed. Finally the dish was returned to the oven for half an hour and was allowed to cool in the desiccators after which it was again weighed. This process was repeated for each of the five samples.
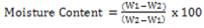
where: W1 = weight of the cool dish, W2 = weight of the cool dish with the sample and
W3 = weight of the dried dish and the sample
The results obtained from the study are presented in Tables 1 and 2. Table 1 shows the values of the oil properties observed from the extracted oil from chestnut at different levels of drying temperature. The comparison of the extracted oil with other seed oil is presented in Table 2.
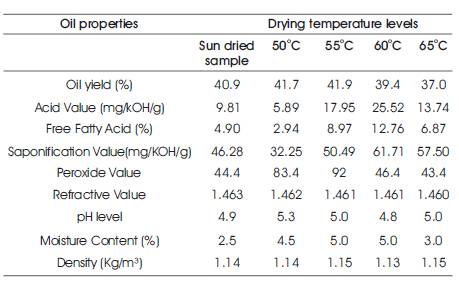
Table 1. Physicochemical properties of Oil Extracted from chestnut
Table 1, shows that, the oil yield from chestnut is highest (41.9%) at drying temperature of 55⁰C. It decreased after this temperature to 39.4 % and 37.0 % at 60⁰C and 65⁰C drying temperature respectively. Comparing these values with other seed oil as given in Table 2, the oil content of the chestnut is higher than safflower (30.5 %), Soybean (21.0 %), corn (4.5 %) and cotton seed (22.9 %) as reported by (Esuoso & Odetokun 1995). However, the value is lower than that of groundnut oil (47.2 %). This shows that chestnut can as well produce similar quantity like Soybean and cotton seed oil. It also indicates that processing of chestnut oil for industrial or edible purpose would be economical.
Free Fatty Acids can stimulate oxidative deterioration of oils by enzymatic and / or chemical oxidation to form off flavor components. Free fatty acid value is an indication of lipase activity (Ukhun 1986). Fatty acid (%) has been calculated based on the molecular weight of the dominant acid. Free fatty acid indicates the possible hydrolytic degradation of the oil and the acid value is employed to ascertain the quality (condition) and edibility of the oils. In Table 1, it can be observed that the free fatty acid from chestnut has its highest value (12.76 mg/g) at drying temperature of 60⁰C. A further increase in temperature resulted in a decrease in FFA. Comparing these values with other oils, as given in Table 2, the free fatty acid from chestnut is higher than soybean (0.5 mg/g), Cucumeropsis Adulis (white melon seed) (0.35 mg/g), Melon (2.38 mg/g), Avocado (0.37 mg/g), Indian bean (0.79 mg/g), corn (1.5 mg/g), cotton seed (0.7 mg/g). The values of free fatty acid obtained are high at 55⁰C, 60⁰C, and 65⁰C drying temperature but lower at sun dried and 50⁰C temperature. The implication of this is that chestnut oil having lower value of free fatty acid is potentially edible while those with higher values need to undergo refining to make it suitable for making them edible and suitable for industrial purposes. Free fatty acid is also related to smoke point. Chestnut with low free fatty acid value will have a high smoke point, so it would be suitable for stir fry cooking (Akintayo and Bayer 2002) .
Saponification value obtained from Table 1, has its highest value of 61.71mg/KOH/g at the drying temperature of 60⁰C. It decreased with an increase in temperature to 57.5mg/KOH/g at 65⁰C. In Table 2, the saponification value from chestnut is lower than Soybean 193.0 mg/KOH/g, Groundnut 193.20 mg/KOH/g, Melon 197.57 mg/KOH/g, Avocado 246.70 mg/KOH/g, Indian bean oil 185.08 mg/KOH/g, Corn 190.6 mg/KOH/g, Cotton seed 195.0 mg/KOH/g. A saponification value of 200mg/KOH/g indicates high proportion of free fatty acids of low molecular weight. This shows that, the oil may not have a potential use in soap making and in cosmetics industry. This property makes them useful as sources of essential fatty acids required in the body (Akanni, M.S., Adekunle, A.S., & Oluyemi, E. A. 2005).
Peroxide Value is an index of rancidity, thus the high peroxide value of oil indicates a poor resistance of the oil to peroxidation during storage (Mohammed and Hamza 2008). The peroxide value of chestnut oil has the highest value of 92mg/KOH/g at a drying temperature of 55oC, 43.4 mg/KOH/g and 44.4 mg/KOH/g at 65⁰C and sun drying temperature respectively. The peroxide value from chestnut is higher than Cucumeropsis Adulis (white seed melon) 2.85 mg/KOH/g, Indian star apple 1.57 mg/KOH/g, as reported by Adebayo et al. (2012). This is also higher than maximum acceptable value of 10 mg/KOH/g set by the ‘Codex Alimentarius Commission’ for such oils as groundnut seed oils. Peroxide value is an indication of level of deterioration of oil (Adebayo et al. 2012) .
The value of refractive index obtained from chestnut oils are similar to those of Groundnut 1.449%, Safflower 1.4750%, Soyabean 1.4730%, Cucumeropsis Adulis (White seed Melon 1.4622%, Melon 1.4680%) and Cotton seed 1.4700%. The higher values of the properties obtained for the crude oils revealed the necessity to purify the oils. The high refractive index of oil also showed that the fatty acids in the oil will contain a huge number of Carbon atoms (Bello and Olawore 2012).
The pH value of the extracted chestnut oil from this study is shown in Table 1 and ranges between 4.8 and 5.3. This shows that, the level of acidity is high.
The moisture content obtained from the current study has highest value of 5.0% and the least value of 2.5 %, as shown in Table 1. The value is lower than groundnut 5.8% reported by (Audu I.O., Oboho, I.O, & Aluyor, E.O 2013). Lower moisture content implies good shelf life characteristics of oil.
The density of the oil obtained from the chestnut is in the range of 1.13 kg/m³ as shown in Table 1. This implies that the oil is lighter than water; hence it will float on water at room temperature. The density of a material is defined as the measure of its mass per unit volume (e.g in g/ml). The density of vegetable oil is lower than water and the differences between vegetables oil are quite small, particularly amongst the common vegetable oils.
The results of the investigation reveal that chestnut oil has the highest yield of 41.9 % at drying temperature of 55⁰C. The highest acid value of 25.52 mg/KOH/g at drying temperature of 60⁰C, The highest Free Fatty Acid (FFA) of 12.76 % at drying temperature of 60⁰C, The highest saponification value of 57.50 mg/KOH/g at drying temperature of 65⁰C, The highest peroxide value of 83.4 mg reac. O₂ /g at drying temperature of 50⁰C and the moisture content of 5.0 % at drying temperatures of 55⁰C and 60⁰C respectively. The color and odor is similar to other common oils such as groundnut and melon. It is concluded from the results obtained that:
Chestnut oil has a very high yield of 41.9% and a good shelf life. It can be produced in commercial quantity. It has a variety of applications including the production of biodiesel.