Figure 1. Manufacture of Vinyl Chloride Monomer
This paper deals with the development of new process simulation modules and related teaching materials that is incorporated in the classes. The process simulation package ‘CHEMCAD’ is used to develop these modules. The main benefit of using a process simulator in encouraging the systems approach is, its powerful interactive aspect that allows students to identify and alter in real-time, physical properties such as pressure and temperature, chemical properties, and other relevant process parameters of a system are being studied. Afterward, the students can examine the resulting changes not only on the particular unit operations but more importantly, on the overall system behavior as well as the operating economics. Similarly, by using a process simulator, the students can imitate controlled 'misbehavior' of the system and in doing so, be able to study, realize, and understand the effects and impacts of the various process parameters on each and every component of the system.
The new modules and teaching material proved to actively engage students' participation and have been included horizontally through time so that the principles of each set modules stresses on are easily mastered from week to week. The modules were developed in such a way that they proceed from simple to complex applications. The section has been integrated vertically so that they can strengthen the subject matter learned by the students in the lecture component of the capstone design course. Vertical integration has been achieved by developing modules that are directly related to the topics the students are currently taking in a particular week. The aim of this paper is to look at the process in detail and to develop a simplified CHEMCAD simulation and create scenarios, which will be used as teaching tools. The chosen chemical process is the manufacture of vinyl chloride monomers. Literature reviews were done first to fully understand the process. A base case design was generated and a generic block diagram with the mass and energy balance for the process was done. They were then implemented into CHEMCAD Software. Finally, it is converted to teaching tools in the form of tutorial/assignments, written in accordance to subject teaching goals. The processes were successfully implemented into CHEMCAD software and converted into teaching tools that illustrate process or structure analysis.
The Chemical Engineering syllabus has undergone incessant revolution to offer students with a precise learning in the fundamentals of chemical engineering. The syllabus also aims at giving students specific training in applications of chemical engineering in the industries. In the University of Pune, the chemical engineering syllabus represents well the broad quality of any reputable curriculum of a well-known University around the globe. As highlighted by the Council for Chemical Research (Armstrong, 2006), the attributes include molecular transformations, multi-scale descriptors, and a systems approach. The motivation of the present work was to focus on the systems component and enhance the training of chemical engineering students in the area of Process Engineering. The project was also aimed at expanding the learning of students in the laboratory. New computer simulation labs and open-ended case studies were integrated within the course. Over 60 students a year are benefiting from this improvement. Students had handson- experience with state of the art simulation software (i.e. CHEMCAD) and were able to conduct more true-to-life engineering simulations of more complex chemical engineering processes. Moreover, working on these reallife industrial case studies enabled undergraduate senior students to understand the importance of the systems approach as an improving educational concept to be adopted in the undergraduate curriculum and to meet AICTE New Delhi requirements. This is in addition to the requirement that graduating students must possess the ability to effectively use computational and process simulation tools necessary for chemical engineering practice.
Process simulators, such as CHEMCAD is being used extensively in chemical engineering design courses. CHEMCAD is one of a powerful engineering simulation tool, has been exclusively created with respect to the program architecture, interface design, engineering capabilities, and interactive operation. It is widely used in universities and colleges in introductory and advanced courses especially in chemical engineering. In industry, the software is used in research, development, modeling and design. This paper illustrates the development of new process simulation modules and associated teaching materials that is integrated within the course. The process simulation package CHEMCAD is used to develop those modules, in order to achieve the aims set forth so far, and that was focused on the following objectives.
In order to meet the above stated objectives, it is been proposed the development of new process simulation modules and associated teaching materials that will be integrated within the course. It was focused on the process simulation package CHEMCAD. Other commercial process simulation software packages today include Aspen, HYSYS, PRO/II with PROVISION, and SuperPro Designer. According to Seider et al. (2004), it is relatively straightforward to switch from the use of one simulator to another, once the principles of process simulation have been understood. The choice of CHEMCAD is based mainly on the existing faculty's expertise.
The main benefit of using a process simulator in encouraging the system approach is, its powerful interactive feature that allows students to specify and modify in real-time, physical properties such as pressure and temperature, chemical properties, and other relevant process parameters of a system being studied. Subsequently, the students can observe the resulting changes not only on the individual unit operations but more importantly, on the overall system behavior as well as the operating economics, which really are the actual aspects emphasised by the systems approach. Likewise, by using a process simulator, the students can reproduce controlled 'misbehaviour' of the system (Mah and Himmelblau, 1996). In doing so, students will be able to study, understand, and appreciate the effects and impacts of the various process parameters on each and every component of the system.
Process simulators have also proven to be effective teaching tools to demonstrate a multitude of chemical engineering concepts, primarily the fundamental core principles of the field. These core fundamentals include: material and energy balances, thermodynamics (in particular, physical and thermodynamic property analysis, estimation, and regression), reactor design, transport phenomena (heat and mass transfer), multicomponent separations, and process flowsheeting (Edgar, 2006). In delivering these modules, the authors followed an approach that was successfully utilized for the teaching of similar modules (Khor et al., 2008). The teaching and learning activities took the form of two% three-hour computer laborator y modules, each structured as follows. In the first hour, the course instructor provides formal instruction on using the simulator, with the students following along on their individual personal computer terminals. Within this setting, the instructor functions both as a facilitator and as a personal tutor by moving around and interacting openly with the students on a one-on-one or small group basis. In instances where there are common problems and misunderstandings faced by the students, the instructor can switch to deliver a mini lecture to address the issue.
Edgar et al. (2006) have highlighted the success of such an integrated lecture. In the subsequent second hour, the instructor supervises hands-on practice for the students and educates them in the difference between the incorrect uses of the software as opposed to an incorrect understanding in formulating solutions to a problem. The final third hour is an optional self-regulated free practice for the students, in which they are encouraged to help and discuss with each other, and in doing so, achieve independent learning-by-doing.
The new modules and teaching material proved to have actively engaged students' participation and were integrated through time, so that the principles of each set modules stressed on are easily mastered from week to week. The modules were developed in such a way that proceed from simple to complex applications. The modules were also integrated vertically, so that they can reinforce the subject matter learned by the students in the lecture component of the capstone design course. Vertical integration was achieved by developing modules directly related to the topics that the students were currently taking in a particular week. This can enable the students to apply materials that they were currently learning to more realistic case studies of the lab modules.
The developed modules and learning outcomes are summarized in the following section. A brief description of each module is provided below.
The manufacture of vinyl chloride, a monomer intermediate for production of polyvinyl Chloride, is an important plastic that is widely used for wire and cables, paper and textile coatings, and other domestic uses. It is considered as a commodity chemical that is produced continuously throughout the world. Vinyl chloride is an extremely toxic substance, and therefore, industrial plants manufacture it or process it as it must be carefully designed to satisfy governmental health and safety regulations (Warren et al. 1999).
Figure 1 shows the block diagram of the process manufacturing of vinyl chloride monomer.
Ethylene, stored as gas in large cylindrical vessels (at 1000 psia and 70ºF ) and chlorine, stored as a liquid (at 150 psia and 70ºF ) is fed into a cylindrical reaction vessel at 363K and 1.5 atm with ferric chloride as a catalyst. The following reaction takes place:

Experimental data has indicated that at the above reactor conditions, 98% of the ethylene are converted to 1,2-dichloroethane.
This unit performs the temperature and phase change operations in the form of a large kettle, with tube bundle inserted across the bottom. Saturated steam is used as a heating medium whereby the dichloroethane liquid is heated to its boiling point and vaporized. The large vapor space is provided to enable liquid droplets, entrained in the vapor, to coalesce and drop back into the liquid pool, that is, to disengage from the vapor which proceeds to the pyrolysis furnace.
This unit performs two operations
It preheats the vapor to its reaction temperature, 773K and it carries out the pyrolysis reaction.

The dichloroethane intermediate is converted to vinyl chloride by thermal cracking. (60% of conversion is assumed).
A quench tank is designed to rapidly quench the pyrolysis effluent to avoid carbon deposition in a heat exchanger. Cold liquid (principally dichloroethane) is showered over the hot gases, cooling them to their new dew point, 443K .
To produce a saturated liquid at 279K, the phase-change operation is carried out by a condenser that transfers heat to a refrigerant.
This consists of two distillation columns whereby the components are being separated. The first column separates HCl from other components, while the second column separates the vinyl chloride from the unreacted dichloroethane. The unreacted dichloroethane is then recycled back for optimization purposes.
An attempt has been made to try to simulate the above vinyl chloride monomer manufacturing process in CHEMCAD simulation software. Figure 2 shows the CHEMCAD model block diagram ( CHEMCAD 5.1 manual ).
The simulation is based on a petrochemical complex that produces 42 tons/hr due to the risen demand for vinyl chloride monomer. The feed rate used in this study is given in Table 1.

Table 1. Amount of feed rate
The main product will be vinyl chloride monomer while the by product is hydrogen chloride. Both have a purity of 99.9% following is the description, assumptions and basis for each block.
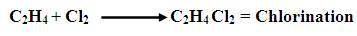
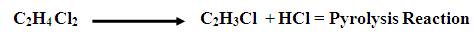
A Process Flow Diagram has been done for the above process. The following abbreviations have been used for the equipment:
The temperature of the chlorination reactor is a very important operating variable as it controls the amount of ethylene being converted to dichloroethane, which in turn, affects the amount of vinyl chloride produced. The temperature of the pyrolysis furnace is another vital variable as it affects the conversions from dichloroethane to vinyl chloride. A quencher unit was required as the rate of carbon deposition was high. However, if on a pilot-plant testing, the measurement of the rate is found to be otherwise (low), it would be possible to implement a design with a feed/product heat exchanger. This may in turn affect the energy balance of the system. The above can then be used in teaching tools whereby an analysis of different conversion rates (for chlorination reactor and pyrolysis furnace) on feed and recycle rates can be investigated. Another useful teaching tool will be to introduce the feed preheater to the process and analyze the savings that can be made.
Build and use Aspen simulations are build and used to answer a proposed method of manufacturing vinyl chloride monomers which is to thermally crack dichloroethane from chlorination of ethylene(John J. McKetta,1976).
The model uses a fresh feed containing chlorine and ethylene in a 2½:1 ratio, contaminated with carbon disulphide (CS2 ) in a 1:500 ratio. This fresh feed of mixture is fed to a reactor operating at 343 – 363K and 1.5atm Figure 3 shows the input-output level diagram of VCM.

Figure 3. Input-output level diagram
The conversions for this reactor range from 20 to 98%, depending on the reaction conditions. The outlet of the reactor will be mixed with the recycled dichloroethane and condensed fully to liquid phase before being pumped to an evaporator. After the evaporator, the vapor goes through the pyrolysis furnace operating at 773 – 788K where the dichloroethane undergoes thermal cracking to form VCM and hydrogen chloride.
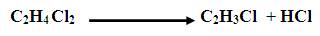
Conversion from 20 to 65% is possible depending on the furnace conditions. The hot stream leaving the furnace is quenched to reduce carbon deposition in heat exchanger and later condensed before entering the distillation column to separate the different components. Hydrogen chloride is first separated from the first column while the second column separates the VCM. A purge stream is introduced to prevent accumulation of unreacted components. The concentration of ethane in the combined recycle plus fresh feed to the reactor must not exceed 0.02% of CS2 . Production of 37.8 tons of VCM (99.9 %wt purity) per hour is required. Following are some questions generated during the study and their answers are also given.
Number of Components, n = 6 (Cl2, C2H4, C2H4Cl2, C2H3Cl, HCl, Cs2)
Number of reactions in B1 and B7, r = 1
| B1: Rstoic | = c + 4 + r = c + 5 |
| B2: Heater | = c + 4 |
| B3: Mixer | = n(c + 2) = 2c + 4 |
| B4: Pump | = c + 3 |
| B5: Heater | = c + 4 |
| B6: Rstoic | =c + 4 + r = c + 5 |
| B7: Heater | = c + 4 |
| B8: Heater | = c + 4 |
| B9: Sep | = 2c + 2 |
| B10: Sep | = 2c + 2 |
| B11: Fsplit | = c + 3 |
Therefore, total = 14c + 40
Interconnecting streams = 11(c + 2) = 11c + 22
Hence, remaining = (14c + 40) – (11c + 22) = 36
Feed = 8 (Pressure, Temperature, all 0% except Cl2, C2H4 and C2H6 )
CompositionB1 (Rstoic) = 3 (Pressure, Temperature, % conversion of C2 H4 )
B2 (Heater) = 2 (Pressure, Temperature)
B4 (Pump) = 1 (Pressure)
B5 (Heater) = 2 (Pressure, Temperature)
B6 (Rstoic) = 3 (Pressure, Temperature, % conversion of C2 H4 Cl2 )
B7 (Heater) = 2 (Pressure, Temperature)
B8 (Heater) = 2 (Pressure, Temperature)
B9 (Sep) = 6 (Top stream = 100% HCl, the rest 0%)
B10 (Sep) = 6 (Top stream = 100% C2 H4 Cl, the rest 0%)
B11 (Fsplit) = 1 (Purge fraction)
Total = 36
Degrees of Freedom = Remaining – Specifications
= 36 – 36
= 0
Therefore, the model is solvable.
A sensitivity analysis was done to show the effects of different conversion rates (chlorination and pyrolysis furnace) to the feed flow rate and recycle stream flow rate.
d. Economic Potential at input-output levelThe prices of the chemicals are given in Table 2.
Economic potential = (Product VCM + ByproductHCL) – (FeedCL2 + FeedC2 H4)

Table 2. Current prices of chemicals
This paper focuses on enhancing students' computational knowledge in the area of process simulation and design. The goal was achieved through the development of new process simulation modules as well as associated teaching materials that were integrated within the chemical engineering course. A module was developed and integrated horizontally and vertically in a way that the chemical engineering principles are stressed and reinforced. In future, additional modules could be added.
It has also been revealed that it is possible to simulate processes using CHEMCAD software. To be as realistic as possible, operating values for each unit is required. Otherwise, heuristic assumptions have to be made.