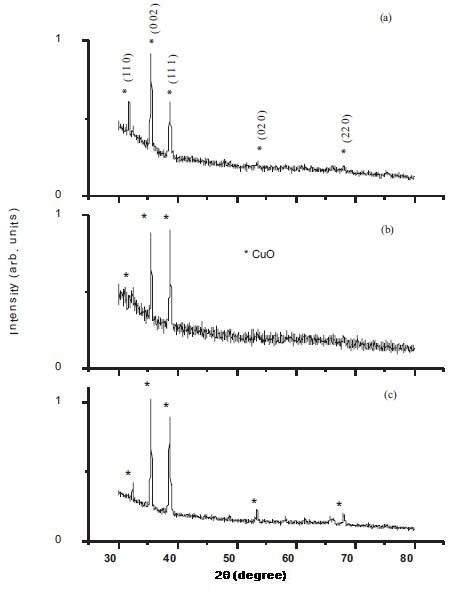
Figure 1. XRD diffractograms of SDS assisted CuO films deposited at nsubstrate temperature of (a) 300˚C :Sf1 (b) 350˚C:SF2 and (c) 400˚C:SF3
In this paper an attempt has been made to study the impact of surfactant on the properties of the ultrasonically spray deposited CuO films. An aqueous solution of cupric nitrate trihydrate (Cu(NO3)2.3H2O)modified with Sodium Dodecyl Sulphate (SDS) surfactant is used to deposit CuO films on glass substrate by Ultrasonic spray pyrolysis technique. The X'Pert Panlytical Diffractometer was employed for the phase identification of the films using Cu Kα radiation (λ = 1.5405 Å, 30mA, 40 kV) in 2θ range from 30-80˚. The Field Emission Scanning Electron Micrographs (FESEM) and EDAX (Energy Dispersive Analysis of X-rays) spectrum were recorded on JEOL JSM-6700F Scanning Electron Microscope with a beam voltage of 30 kV. The depth profiler (Dektek 3030 XT) was employed for monitoring the film thickness and was found to be 400 ± 20 nm. The X-Ray Diffraction (XRD) studies of the films deposited at various substrate temperatures indicate the formation of monoclinic CuO with preferential orientation along the(002) plane for all samples. Surfactant modified films showed an increase in crystallite size of 35 nm at substrate temperature of 300 ˚C. The Scanning Electron Micrograph (FESEM) confirms the uniform distribution of facets like grains on the entire area of substrate. The results obtained in this study illustrate that SDS modified films show a significant reduction in the particle agglomeration thereby increasing the surface to volume ratio which in turn improves their sensing performance.
Considerable efforts have been devoted to characterize and describe the physical, chemical properties of metal oxide due to their important applications in many technological fields (Ueda, Maeda, Hosono & Kawazoe,1998, Liu, Zhang, Wang, Gao, Li, Zheng, Ringer, Zhang & Zhang, 2007, Zhu, Zhao, Pan, Zhang, Fan, Zhang & Xiao, 2007, Ferreira, Tabacniks, Fantinia, Fariab & Gorensteinb, 1996). CuO with a monoclinic crystal structure, is an important p-type semiconducting material and has been utilized in different technological areas (Huanga, Yanga, Lia, Gua, Dua, Lub & Shi, 2004, Kari, Brown & Choi, 2006, Serin, Serin, Horzum & Celik 2005, Maruyama, 1998, Santra, Sarkar, Mukherjee & Ghosh, 1992, Drobny & Pulfrey, 1979). CuO films have been deposited by various researchers using different techniques (Muthe, Vyas, Narang, Aswal, Gupta, Pinto, Kothiyal & Sabharwal, 1998, Salarian, Hashjin, Shafiei, Salaria & Nemati, 2009, Pradhan, Sarkar, Sinha, Basu & Pal, 2010, Alamolhoda, Seyyed Ebrahimi & Badiei, 2006, Zhang, Wang, Li, Chen, Qian & Zhang, 2006, Liu, Chu, Li & Dong, 2006, Bedi & Singh, 2010).
Surfactants can play an important role in synthesizing the material in different interesting morphologies (Kose, Atay, Bilgin & Akyuz, 2009, Perednis & Gauckler, 2005, Lv, Wang, Zhou, Jing, Wu & Zhao 2009). Surfactant molecules may be used to control the size, shape and agglomeration among the particles. Surfactants have the tendency to absorb on the specific crystal planes causing an anisotropic growth of the crystal structure. The addition of surfactant reduces the surface tension of the precursor solution, which facilitates nucleation and allows its easier spreading. Surfactant with molecules composed from a hydrophilic head and a hydrophobic tail into precursor results in the formation of reverse micelles in the gel. Hydrocarbonic tail length and the formation of reverse micelles control the growth and the distance between the particles and agglomeration( Elansezhian , Ramamoorthy, Kesavan & Nair 2009). SDS has been extensively used in synthesizing CuO by different routes (Xu, Xu, Liu & Wang, 2002, Xu, Qin, Yang & Li, 2003, Liang, Peng & Wang, 2005). Most of the studies involve the synthesis of CuO powder. However, limited data is available concerning the effect of surfactant on the aerosol spray deposited CuO thin films.
In this study CuO films have been deposited by ultrasonic spray pyrolysis technique with an aim to observe the effect of SDS addition on the structural and morphological properties of the CuO films. The changes induced on the structural morphological properties of CuO films by the surfactant addition helps to utilize them on some technologically important areas such as in solar cell fabrication and gas sensors.
The films of CuO were deposited onto glass substrates by using 0.2M aqueous solution of trihydrated cupric nitrate (Cu(NO3).3H2O). To prepare 0.5 M aqueous solution of SDS, required quantity of salt has been dissolved and 10 mL of it was added to aqueous cupric nitrate solution. The resulting solution was stirred vigorously for 4 hours to form a homogeneous solution. The solution so prepared was used to generate an aerosol using an ultrasonic nebulizer (Omron Make NE-U17) which was subsequently transferred on the ultrasonically cleaned, preheated amorphous glass substrate using air as carrier gas. The preparative parameters of the ultrasonic spray setup such as nozzle to substrate distance, solution concentration, solution spray rate etc. were optimized to obtain pin hole free and adherent films of CuO. The substrate temperature was varied from 300 to 400˚C ± 5 ˚C in a step of 50 ˚C which is controlled by thermocontroller (DTC Selec 303). The substrates were heated to required temperature for film deposition by an electrical heater. The distance between the nozzle and the substrate was maintained at 25 cm.
The phase identification of the film was performed by XRay Diffraction (XRD) on X'Pert Panlytical diffractometer using Cu Kα radiation (λ = 1.5405 Å, 30mA, 40 kV) in 2θ range from 30-80˚. To study the surface topography and composition analysis of copper oxide films, Field Emission Scanning Electron Micrographs (FESEM) and EDAX spectrum were taken on a JEOL JSM-6700F Scanning Electron Microscope with a beam voltage of 30 kV. The thickness of the film was monitored using Depth Profiler (Dektek 3030 XT) and was found to be 400 + 20 nm.
XRD patterns of as deposited CuO films with SDS at different substrate temperature are shown in Figure 1. The presence of intense peaks in the XRD patterns of the films shows that the CuO films are polycrystalline in nature. Two most prominent peaks can be clearly seen at 2θ value 35.5˚ and 38.7˚ corresponding to atomic planes (002) and (111) respectively of CuO phase. No peak corresponding to Cu2O phase of copper oxide has appeared in the XRD pattern which indicates the formation of pure CuO films. The surfactant addition in the precursor improves the crystallinity and indicates the enhanced growth of crystallites along certain preferred directions as evident in Figure. 1(b, c). ITO film deposited by sol gel method in the presence of surfactants shows that surfactant acts as important factor in controlling the preferred growth orientation of ITO (Indium Tin Oxide) films (Jiaxiang, Da, Nan & Yue, 2010). Thus surfactant might help to self assemble the grains in the minimum energy growth direction. The micelle which directed the growth thermally decompose on the substrate and leads to an oriented growth.

Figure 1. XRD diffractograms of SDS assisted CuO films deposited at nsubstrate temperature of (a) 300˚C :Sf1 (b) 350˚C:SF2 and (c) 400˚C:SF3
To determine the preferential orientation in CuO films with and without the addition of surfactant from the XRD data, the texture coefficient was calculated using the equation below
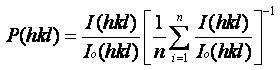
where Io represents the standard intensity, I is the observed intensity of the (hkl) plane and n is the reflection number. Calculated P(hkl) values reveals some important structural information. For the preferential orientation P(hkl) has to be greater than one (Kose, Atay, Bilgin & Akyuz, 2009). It has been observed that P(hkl) value equals to one for all the reflecting planes indicating that films are randomly oriented. P(hkl) values greater than one indicate the abundance of the grains in a given (hkl) direction. For the P(hkl) values that lie between zero and one there is lack of grain orientation in that direction (Perednis & Gauckler, 2005). The diffraction parameters for the deposited CuO films have been listed in Table 1. As can be seen from Table 1, structural parameters depend on the synthesis parameters. Texture coefficient values greater than one for the peak located at 2θ value corresponding to the reflection from atomic plane (002) of the films reveal the preferential orientation of the film.
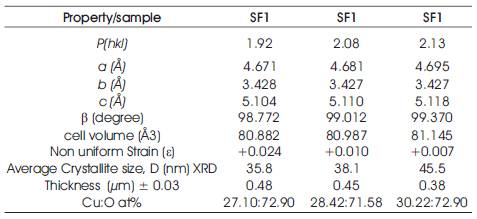
Table 1. Lattice parameters, strain and crystallite size, elemental composition
The average grain size and the internal lattice strain of the film samples have been evaluated by the Hall-Williamson equation expressed as:
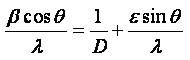
where β is the FWHM (Full Width at Half Medium) of the powder, θ is the Bragg angle, λ is the wavelength of X-ray used, D is the grain size and ε is the internal strain. The D and ε values were calculated from the least square fit to β cosθ/λ vs sin θ/λ plots for the prominent peaks of the samples as shown in Figure 2. The intercept of the Hall equation plot on the y-axis give rise to the average grain size and corresponding values are recorded in Table 1. It has been observed that the addition of SDS in the precursor sol results in an increase of the average grain size. The crystallite size around 35 nm has been observed in case of the CuO film with 0.5M SDS deposited at substrate temperature of 300˚C, thus indicating the formation of nanocrystalline phase.
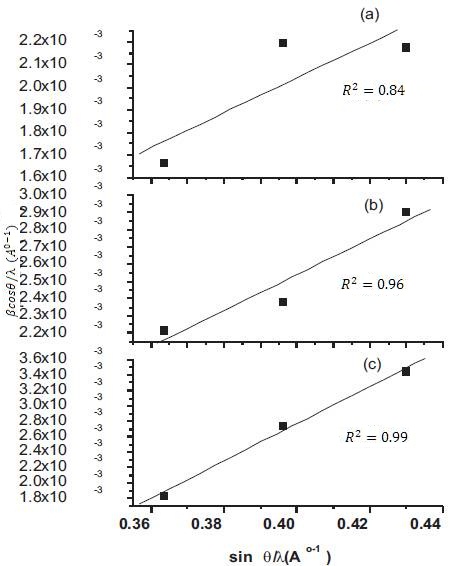
Figure 2. Hall-Williamson equation plot of SDS assisted CuO films (a) SF1 (b) SF2 and ( c) SF3
A positive slope has been observed in the Hall equation plot for CuO films deposited with SDS confirms the presence of tensile strain in the crystal lattice (Perednis, Gauckler, 2005). The magnitude of the slope in SDS assisted CuO film show a decrease which suggests the decrement of the strain. Since strain is the indication of defects in the film, the drastic decrease in strain from 0.024 to 0.007 with substrate temperature in the SDS assisted grown films, suggests the formation of high quality films with improvement in crystallinity and crystallite size.
To find out the effect of surfactant and substrate temperature on the CuO films, the lattice parameters ( a ≠ b ≠ c, α = γ = 90˚ ≠ β for monoclinic structure ) and the volume of unit cell were calculated using the relations
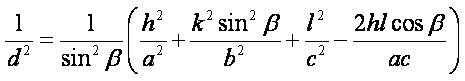
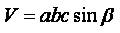
where d is the interplanar spacing, h,k,l are Miller indices of the crystal planes, a, b, c, β are the lattice parameters and V is the volume of the unit cell. The values of the lattice parameters are in good agreement with those of the ICDD card 41-254. The change in the lattice parameters and the unit cell volume with the surfactant assisted grown films gives the evidences of the strain. Variation in the lattice constant can also be observed with the addition of surfactant and substrate temperature as shown in Table 1. The origin of strain in the films is also related to the misfit of the lattice which in turn strongly depends upon the deposition conditions. Dislocations are the imperfections in the crystal lattice associated with the misfit of the lattice in the crystal in one part from the other part. It is an important parameter that governs the growth mechanism. Dislocations are mostly non uniform in nature and depend upon the crystallite size.
The film samples have been characterized for morphological and elemental composition by FESEM and EDAX techniques. SEM micrographs were taken to investigate the effect of SDS on the surface morphology of the CuO films and obtained micrographs are shown in Figure 3. The SEM micrographs show that the films deposited with surfactant are smooth, void or crack free and showing self assembling with nano-sized particle agglomerates into pyramid like grains. The size of the particles which form those agglomerates, is much smaller than the grain size and is difficult to resolve. The film texture is smoother for the films with smaller grain size. It was observed that SDS assisted CuO thin films deposited at 300˚C substrate temperature were smooth, dense and showing a uniform distribution of grains. The contrast of the images shows that when SDS was added, the particle size significantly reduces till 45 nm, while the particle sphericity was improved. Thus SDS assisted CuO films showed low agglomeration. This might be due to the adsorption of surfactant molecules absorbed on the particle surface which acts as a spacer among the neighbouring particles and prevent the coalescence and the formation of hard agglomerates of the individual particles. The formation of loosely agglomerated structures on the film surface help to improves their adsorption properties. The increase in adsorption helps to improve the gas sensing properties of the films. Thus surfactant assisted USP deposited films are potential candidates in the field of gas sensing technology.
The EDAX spectrums of the film samples are shown in Figure 4 and elemental composition of the samples has been tabulated in Table 1. The analysis shows that the SDS assisted grown films are metal deficient. The elemental composition taken at different location of the film sample shows that content of oxygen increases with an increase in substrate temperature. The increase in oxygen content leads to more interaction with photon or any other toxic gas molecules by physical or chemical bonding. This bonding induce changes in conductivity of films hence affecting the photosensitivity or gas sensitivity properties.
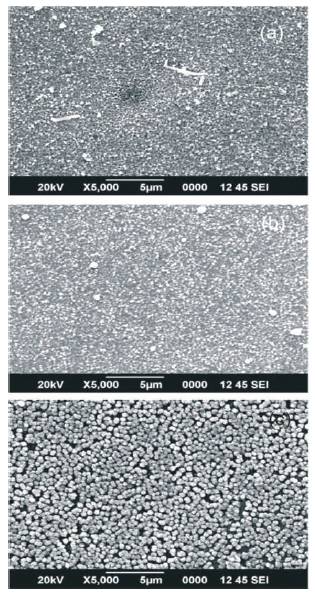
Figure 3. FESEM images of SDS assisted CuO films (a) SF1 (b) SF2 and ( c) SF3
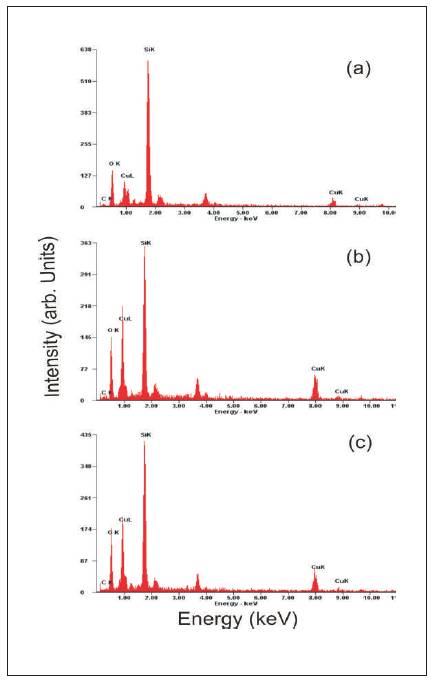
Figure 4. EDAX spectrum of SDS assisted CuO films (a) SF1 (b) SF2 and ( c) SF3
The nanocrystalline CuO thin films has been deposited on glass substrate by a surfactant assisted Ultrasonic Spray Pyrolysis (USP) method. The crystallite size in CuO has been found to be around 35 nm with the addition of 0.5M SDS. The results indicate that CuO nanocrystallites are subjected to considerable strain and it decreases with the addition of SDS in the solution. The faceted type distorted spherically shaped CuO nanoparticles with minimum agglomeration are obtained in all film samples. The affinity of CuO nanopaticles on the film surface appears to enhance for foreign particles with addition of surfactant. An oxygen enrichment of the film samples makes them more suitable towards sensor technology at room temperature. Thus, the study reveals that the properties of synthesized CuO nanoparticles can be controlled by the addition of surfactant as well as with substrate temperature.
The authors wish to thank Director IIT Roorkee, STIC, Kochi and RSIC, Punjab University, Chandigarh for providing FESEM, EDAX and XRD facilities.