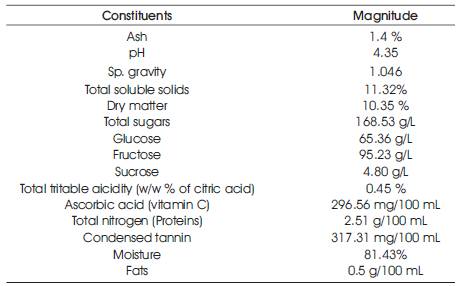
Table 1. Physico-chemical analysis of cashew apple juice
The use of cashew apple juice to produce biofuel-alcohol by biotechnological process represents an important alternative to avoid wastage of large quantity of the fruit. Faced with the challenges of transforming raw materials and given the large amounts of cashew fruits destroyed at the expense of cashew nuts, a study of the fermentation of cashew apple juice is required for economic utilization of the agro-residue. This paper focuses on the alcohol fermentation of cashew apple juice by inoculation with yeast Saccharomyces cerevisiae in CSBR. The optimum parameters for the bioethanol fermentation are time, 96 h; pH, temperature, 55ºC; stirring speed, 35 rpm; cashew apple juice concentration 400 g/L. Maximum yield of ethanol was found to be 0.493 g/g of cashew apple juice at the optimum parameters. The sp. yeast growth rate (µ) was 7.05 s-1 and maximum sp. yeast growth rate (µMAX) was 14.10 s-1 using Monod Model respectively. The enzyme kinetic parameter (Km) was 160.5 g/L for ethanol fermentation at optimum bioprocess conditions. Kinetics of ethanol fermentation from cashew apple juice shows a good agreement with the first order rate kinetics.
The cashew tree (Anacardium occidentale) is native to the northeastern coast of Brazil where it was cultivated by indigenous people long before its discovery by the Portuguese, who introduced the latest in their African colonies and Asia. The value of this tree has focused on cashew that is the subject of international trade and which gives the cashew notoriety. Cashew is an important nut crop which is grown in India on an area of 850 000 ha, producing about 600 000 tonnes/year of raw nuts. The cashew apples or cashew fruits weight is about 5 to 10 times as much as the nuts. It is eaten fresh, cooked in curries, or fermented into vinegar as well as an alcoholic drink. It is also used to make preservatives, and jams in some countries such as India and Brazil. In many countries, particularly in South America, the cashew apple is used to flavor drinks, both alcoholic and nonalcoholic. The cashew apples juice is a stringent and somewhat acrid due to tannin content in the red and yellow varieties and an oily substance, so it is pressuresteamed for 5 to 15 minutes before candying or making into jam or extracting the juice for carbonated beverages, syrup or wine[1-3].
Apart from the cashew nut, the apple is fairly valued except in India and Brazil, especially in the form of juice. In most cashew producing countries, the cashew apple rots in the soil at the expense of nuts. Yet several studies have shown the richness of the apple juice that contains minerals, three to six times more vitamin C than orange juice and ten times more than pineapple juice. The cashew apple juice is rich in tannin and polyphenols, which gives it antioxidant properties and makes it an effective remedy against chronic dysentery; used as a coadjutant in the treatment of premature aging of the skin and relieve scurvy due to its high level of vitamin C. Cashew apple juice enhances fat oxidation during exercise and may enhance endurance performance. Despite the wealth of this fruit and its medicinal effects on health, the cashew apple and its juice are unknown majorly to the public domain [1-7].
So, faced with the challenges of transforming raw materials and given the large amounts of cashew fruits destroyed at the expense of cashew nuts, a study of the fermentation of cashew apple juice is required for economic utilization of the agro-residue. The present study was undertaken for aerobic ethanol fermentation from cashew apple juice in a Continuous Stirred Batch Bio Reactor (CSBR) using yeast S. cerevisiae. Attempts were also made to optimize bio-process parameters [8-11] like time, pH, temperature, stirring speed (rpm) and cashew apple juice concentration (g/L) for maximum ethanol fermentation. The sp. yeast growth rate (µ) and maximum sp. yeast growth rate (µmax) were determined by Monod Growth Model. Enzyme Pyruvate decarboxylase secreted from S. cerevisiae was assayed in the studies. The sp. enzyme activity (v ) and maximum sp. enzyme activity (vmax) were determined using ‘Michaelis-Menten Enzyme Kinetic Model’. The rate constant (k) was calculated for biochemical reactions for ethanol fermentation process in CSBR.
The freeze-dried (lyophilized) yeast S. cerevisiae (MTCC 170) was collected from Microbial Type Culture Collection and Gene Bank, Institute of Microbial Technology, Chandigarh, India, and then it was stored in a freezer at – 4°C. The slant culture was aseptically prepared in growth medium with the ingredients of yeast extract, 3 g; peptone, 10 g; dextrose, 20 g; agar, 15 g; distilled water, 1 L. The medium, after shaking, was sterilized at 15 psi for 15 min in an autoclave. The slant culture was kept for a period of 7 days in an incubator at 30°C for sufficient sporulation. Spore crops were then harvested by washing the fullygrown slants with sterile distilled water and transferred to suspension culture media in Erlenmeyer flasks (250 mL). It was again kept in an incubator at 30°C for 7 days for proper growth. The suspension culture was filtered through several layers of sterile absorbent cotton and cultured again in the same suspension culture media. The final yeast population was arbitrarily chosen as 5.87x106 numbers of cells per mL so that, at the moment the cells were harvested, the culture was growing exponentially. It is important to use cells from an early-logarithmic-phase culture to get reproducible results. The same yeast suspension culture was used in the present studies [8-11]. Cell count was made on a hemocytometer (M/S Perkin- Elmer, Lambda Bio- 40) after a brief treatment with 5 mM galactose, which was found to produce a uniformly dispersed suspension. The following constituents were used as suspension culture media [8-10] preparation per liter which are KH2PO4 - 20g, MgSO4 .7H2O- 5.0g, CaCl2 - 1.0g, MnSO4 . 7H2O- 0.05g, FeSO4 .7H2O- 0.10g, CaCl2 . 6H2O-0.10g, AlK(SO4)2 .12H2O - 0.01g, Na2MoO4. 2.H2O- 0.01g.
The plant fruits, cashew apples consisting of red varieties were collected from local (Kerala) gardens (sample collection and analysis were done during the fruiting seasons). Ripe and intact red cashew apples were refrigerated (– 8 °C) in the laboratory for juice extraction and analysis. Fully mature cashew apples (fully mature apples will detach with a tap on the fruit) which were refrigerated in the laboratory. were detached from the nuts and were washed thoroughly with clean water. Then the apples were cut into small pieces and ground in mixer. The mash was squeezed to extract the juice with the help of an extractor. The juice thus obtained, was filtered through a 0.5 mm mesh sieve. The extracted juice was heated for 15 min in a boiling water bath after adding gelatin at 50 mg/100 ml. Sodium benzoate (0.7 g/L) was added as a preservative after cooling at room temperature (25–30°C) and the juice was allowed to stand overnight at 4°C. After centrifuging, the clarified juice was again filtered through 0.5 mm mesh sieve, followed by physico-chemical analysis which is shown in Table 1 of cashew apple juice. Yet, several factors such as species, growing region, climate, cultural practices, maturity at harvest, the storage atmosphere, and storage conditions, are known to affect the physico-chemical composition of cashew apple juice [12].
The pH was determined using a pH-meter. The calibration of the instrument was ensured by the use of two buffer solutions at pH 7 and 4. It was systematically done before the pH measurements. The measurement was done by immersing the electrode in 5 mL of juice sample.
The TSS was determined by the method by using a refractometer type ATR-W2 plus (2009/230, U K) equipped with a digital display (S/N: 32853, U K).
The total titratable acidity (% citric acid) was determined by placing 10 mL of juice in a beaker and titrating with a solution of 0.1 N sodium hydroxide to pH 8.2 ± 0.1 .
The ash content was determined by the method of incineration. A volume of 15 mL sample was poured into a pre-weighed crucible. The whole was weighed and placed in muffle furnace and the temperature gradually increased. After 3 h, the temperature was set at 550 °C upto 4 hours. The crucible was then cooled in a desiccator and then weighed again.
The concentration of D-glucose was determined before and after the hydrolysis of sucrose by β- fructosidase (invertase). The content of D-fructose from cashew apple juice was determined subsequent to the determination of D-glucose, after isomerization of the D-glucose by Phospho-glucose isomerase. The concentration of Dsucrose was determined by the method of BIS. The total sugars were assayed by the phenol sulfuric acid method (BIS).
Total nitrogen was determined by volumetric after digestion and distillation method of Kjeldahl which takes place in two stages. In the presence of catalyst (CuSO4), organic matter was digested with sulfuric acid. Ammonia was formed and released by sodium hydroxide, was distilled and collected in boric acid solution. Ammonium borate was determined by sulfuric acid solution.
20 mL of juice was added to 80 mL of acetone to yield 80% aqueous acetone solution and shaken for 10 min at room temperature. The proanthocyanidins content was measured according to the vanillin-HCl (Hydro Choloric Acid) method ( 0.5 mL of extract was added with 3 mL of 4% vanillin (w/v) in methanol and shaken vigorously. 1.5 mL of concentrated HCl was immediately added and the tube shaken again). Absorbance was read at 500 nm after being allowed to stand for 20 min at room temperature. The results were expressed as catechin equivalents (mg/100 mL) of the fresh juice [12].
20 mL of cashew juice was added to 30 ml of citric acid 3% (w/v). The mixture was homogenized and centrifuged at 1500 rpm for 25 min at 0 °C. The supernatant was collected and filtered through Whatman paper No 4 and then through a 0.45 mm Millipore membrane. The samples thus treated were stored at –20 °C until analysis in HPLC [12]was completed.
The HPLC (High Performance Liquid Chromatography) equipment (M/S Shimadzu Corporation, Japan) consists of a pump (Shimadzu LC-6A Liquid Chromatograph), an UV spectrophotometry detector (Shimadzu SPD-6A) and an integrator (Shimadzu CR 6A Chromatopac). Chromatographic separation of vitamin C was performed with a column (RP 8, 250 mm × 4.6 mm) maintained at 35 °C using an oven (Meta Therm TM). The eluent was a solution of acetic acid/acetonitrile (85:15) with a flow rate of 1.3 mL/min and the detector was set at 265 nm. A volume of 20 μL of each centrifuged and filtered sample was injected for analysis. The standard solution (without vitamin C) was filtered and injected separately and the analysis was performed in triplicate to get concurrent result. Vitamin C was identified and quantified by comparison of their retention time and peak area with those of standard solution [12].

Table 1. Physico-chemical analysis of cashew apple juice
The fermentation of cashew apple juice[8-11] was performed by inoculation with yeast S. cerevisiae. Experiments are carried out in 3 L batch reactor (conical flask) containing 100g/L of cashew as substrate to be fermented. Suspension culture (1.0 L) as inoculum was added to the reactor. Inoculum is taken from a seven days old suspension culture. 1.0 L of suspension culture media was added to the reactor contents. The initial pH of feed in reactor was maintained at 2.0 by using 0.1 NH2SO4 acid and/or 1 (M) CaCO3 slurry. The temperature of the feed is maintained at 40ºC by means of heating the coil fitted with the off-on temperature controller. The temperature of reactor was measured by a thermocouple. The initial stirring speed was maintained at 20 rpm by inserting magnetic stirrer in the reactor. The mouth of the reactor was fitted with nonabsorbent cotton. Aliquots (Biological term defined as mixture of microbes and other fermented products) were withdrawn every 24 h to monitor alcohol fermentation. Once the fermentation process was completed, cells were removed by filtration and centrifugation and the products were stabilized under refrigeration (– 8 °C) for a period of 3 days. The stabilized products were stored in the glass bottles and pasteurized at 60 ± 5°C for 30 min. Fermented and pasteurized product was analyzed for ethanol concentration (v/v) by gas chromatograph [13-14].
The general method of fermentation was performed at regular intervals of time (24, 48, 72, 96, and 120 h), for various pH (2, 3, 4, 5, and 6) and for different temperatures (40, 45, 50, 55 and 60 °C) respectively. The method was also repeated for different stirring speed (20, 25, 30, 35 and 40 rpm) and for various apple juice concentrations (100, 200, 300, 400 and 500 g/L) respectively. The ethanol concentrations were measured for different variable parameters, while other conditions were kept constant at optimum level (time 96 h, pH 5, temperature 55°C and stirring speed 35 rpm).
The gas chromatograph (Chrom Pack Model CP 9001), equipped with a flame ionization detector and a CP-9010 automatic liquid sampler was used. Data handling was done with the Maestro Chromatography Data System. The injection port of the chromatograph was installed with a hand-made glass liner (length 8 cm, o.d 6 mm, i.d 3 mm). This liner, which acted as a pre-column to prevent the contamination of the gas chromatographic column with nonvolatile material from fermentation process, was stoppered with a dimethylchlorosilane treated glass wool plug and partly filled with small glass beads with a diameter of 1 mm. The fermented ethanol was injected by means of a 50 μL Hamilton syringe (Model 1705, Chrompack) with a removable needle (needle gauge 22 S), penetrating the glass beads by at least 1.5 cm. Injection by <1.5 cm beneath the surface of the glass beads mostly resulted in a broad tailing peak for ethanol. The plunger of the syringe had a Teflon tip to provide an inert leak-tight seal. For routine analyses, 2 μL injections were performed. The liner was replaced within seconds by a new one after some 50 injections ( 2 μL) of ethanol.
The operating conditions were as follows: column 2 m ×2 mm i.d, glass packed with 10% SP 1200/1% H3PO4 on 80/100 Chromosorb WAW, column temperature 120 °C, injection port temperature 200 °C, detector temperature 180 °C, detector output attenuation 27, carrier gas N2 20 mL/min, H 30 mL/min and air 300 mL/min. Freshly packed columns were conditioned overnight at 190 °C with a flow of carrier gas N2 , before being connected to the detector. A few 1 μL injections of 10% formic acid were made to clear the column from unknown impurities. When using a new liner, two 2 μL injections of distilled water were made to clear the new glass beads inside the liner of some unknown impurities. The time to replace the liner, to stabilize the system, and to decontaminate the new liner took ~3 min. For 1 mL samples of water, fermented ethanol of the concentration is 1, 2, 4, 6, 8, and 10 μL was added separately to this stock calibrator, resulting in solutions of 0.5, 1, 2, 3, 4, and 5 g/L respectively. An aqueous stock calibrator of ethanol was prepared with a concentration of 50 g/L. This solution was stored at 4 °C. This aqueous stock calibrator was used for daily calibration.
The fermented solution was filtered and taken in a clean dry test tube [15,16]. It was incubated after shaking for 2 hr at room temperature and allowed to settle. It was centrifuged (500 rpm) for clarification. The supernatant liquid was assayed for Pyruvate decarboxylase (EC4.1.1.1) with 50 mM pyruvate, 5 mM cysteine, 0.03 mM NADH, 0.25 mM thiamine pyrophosphate, and 1 unit of Alcohol dehydrogenase, using triethanolamine hydrochloride 17 (pH 7.4) as buffer. The reaction was initiated with pyruvate. Absorbance was measured for the mixtureat 340nm for 1 min using UV-V i s Spectrophotometer (M/S Perkin-Elmer, Lambda Bio- 40). It was compared with blank reading. One unit of enzyme activity is defined as 1 mol of substrate converted per min.
The yield of ethanol was found to be proportional to the fermentation time. The yield of ethanol increased with the increase in time up to 96 h and then it declined as shown in Figure 1. Maximum yield of ethanol was 0.355 g/g of cashew apple juice at 96 h of fermentation time. After 96 h of time, the yield of ethanol decreased using S. cerevisiae in CSBR. Fermentation time of 96 h is therefore taken as optimum for ethanol fermentation from cashew apple juice with S. cerevisiae.
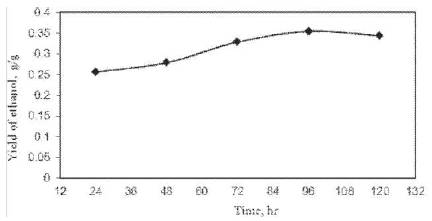
Figure 1. Effect of time on ethanol fermentation
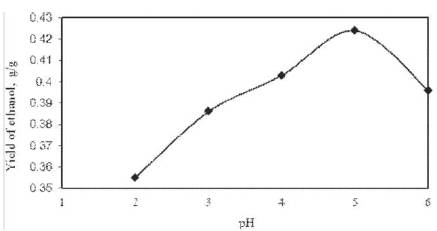
Figure 2. Effect of pH on ethanol yield
The increase in the yield of ethanol was observed with the increase in initial broth pH upto 5.0 and then it declines as shown in Figure 2. Maximum yield of ethanol was found to be 0.424 g/g of cashew apple juice at pH of 5.0 at optimum (96 h) fermentation time. With the increase in pH (>5.0), the yield of ethanol sharply decreased (Figure 2). Initial pH (5.0) was, therefore, taken as optimum for the ethanol fermentation using S. cerevisiae in CSBR. When pH differs from the optimal value, the maintenance energy requirement of the cells increase, death rate increse, that leads to decrease in ethanol fermentation.
With the increase in fermentation broth temperature, the yield of ethanol increased up to 55ºC and then it decreased as shown in Figure 3. Maximum yield of ethanol was found to be 0.472 g/g of cashew apple juice at 55ºC at optimum (96 h) time. Operating temperature of 55ºC was, therefore, taken as optimum for further study. With the increase in temperature (>55 ºC), yield of ethanol declined. Temperatures below the optimum (<55 ºC) depress the rate of metabolism of yeast cells. The higher the optimal temperature (>55 ºC), the less is the growth rate and finally thermal death occurs. At high temperature (>55 ºC), death rate exceeds the growth rate, which causes a net decrease in the concentration of viable yeast populations with lower generation of ethanol. It is, therefore, not surprising that a multitude of physical and chemical parameters may cause perturbations [17] in protein–geometr y and structure of enzymes particularly.
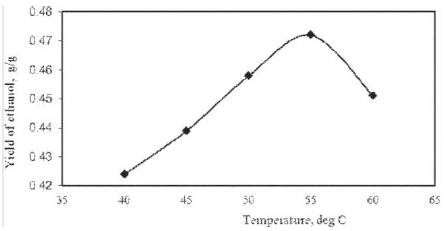
Figure 3. Effect of temperature on ethanol yield

Figure 4. Effect of stirring speed on ethanol yield
With the increase in stirring speed, the yield of ethanol increased up to 35 rpm and then it decreased as shown in Figure 4. Maximum yield of ethanol was found to be 0.529 g/g of 35 rpm at 96 h of fermentation respectively. Stirring speed of 35 rpm was, therefore, taken as optimum for ethanol fermentation with yeast S. cerevisiae in a CSBR. With the increase in stirring speed (>35 rpm), the yield of ethanol was declined. Increase in stirring speed can disturb the elaborate shape of enzyme to such a degree that the denaturation of the protein occurs[8-11, 17] which deactivates the enzymes. Therefore, the yield of ethanol was decreased with increase in stirring speed beyond optimum value.
With the increase in cashew apple juice concentration, the yield of ethanol increased up to 400 g/L and then both decreased as shown in Figure 5. Maximum yield of ethanol was 0.558 g/g at concentration of 400 g/L at 96 h of fermentation. Cashew apple juice concentration of 400 g/L was therefore taken as optimum for ethanol fermentation using yeast S. cerevisiae in CSBR. Low yield of ethanol with increase in concentration can be attributed to product ethanol and substrate (cashew apple juice) inhibition effect. Some enzymes are produced from yeast, whereas others are influenced [8-11, 17] by substrate. The repression and depression processes allow S. cerevisiae to regulate their enzyme (Pyruvate decarboxylase) content in direct response to the environment.
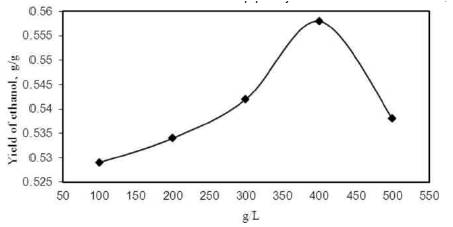
Figure 5. Effect of juice concentration on ethanol yield
The sp. growth rate (= no. of cells/mL. s) of yeast S. cerevisiae for cashew apple juice at different concentration loadings in the optimum bioethanol fermentation parameters in bioreactor was calculated from the respective growth data as plotted in Figure 6 against cashew apple juice (substrate ) concentration to analyze Monod model [17], as shown below,
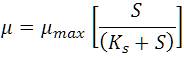
Where, Ks is the kinetic parameter at which the sp. growth rate (µ) is half of maximum growth rate ( µmax), i.e. = max/2, at Ks = S (upto linear portion of the curve). The model indicates a division between the lower concentration range where µ is strongly (linearly) dependent on S, and the higher concentration range where it becomes independent of S (curve portion); here, S is the concentration of the substrate cashew apple juice.
With the increase in juice concentration, the sp. growth rate of S. cerevisiae is first increased, and then it decreases due to the substrate and product inhibition effect. For Monod model, the sp. growth rate (m) of yeast is found to be 7.05 s-1 (upto linear portion of Figure 6) and maximum sp. growth rate (µmax) is calculated as 14.10 s-1 for cashew apple juice fermentation respectively. The kinetic parameter (Ks = S) is determined as 180 g/L (upto linear portion of Figure 6) for cashew apple juice fermentation in CSBR.
An important case of decrease in yeast growth is because of product ethanol concentration and substrate. The utilization pattern of substrate cashew apple juice is significantly influenced by the adaptation characteristics of yeast culture. Adaptation of yeast significantly affects the sp. growth rate, length of lag - phase and overall fermentation of bioethanol. Though the fermentation media contains many numbers of sugars and culture media also contains other carbon sources, [8-11, 17] yeast shows diauxic behavior, where as in the presence of two or more carbon sources, yeast first utilizes the preferential one upto exhausted, then utilize second or other carbon source. They do not utilize both carbon sources at a time. The diauxic inhibition has an effect on sp. growth rate as well as ethanol fermentation with S. cerevisiae.
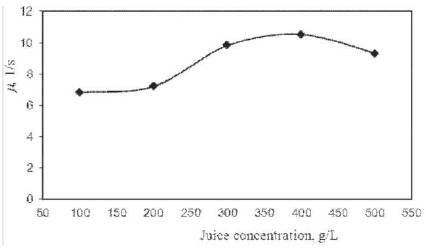
Figure 6. Monod growth model of alcohol fermentation
The enzyme-substrate (Pyruvate decarboxylase–cashew apple juice) interaction varies from one enzyme-substrate complex to another. The Pyruvate decarboxylase activity from yeast S.cerevisiae for ethanol fermentation at various cashew juice concentrations under optimum fermentation conditions is plotted. For the analysis of enzyme kinetic model, sp. Pyruvate decarboxylase activity ( v= Units/mL. min) is plotted against cashew apple juice concentrations by using Michaelis-Menten enzyme kinetic model which is given as below,
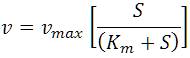
Where Km is the intrinsic kinetic parameter where the substrate (cashew apple juice) concentration (S) at which the sp. enzyme activity (µ) is half of maximum sp. enzyme activity (µmax) i.e. = max/2, at Km = S. The model indicates a division between the lower concentration range where it is strongly (linearly) dependent on S, and the higher concentration range where it becomes independent of S. max is solely a function of rate parameters and is expected to change with the temperature or pH.
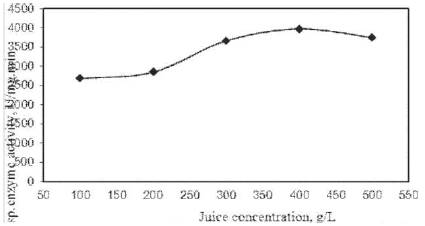
Figure 7. Michaelis-Menten enzyme kinetic model
With the increase in cashew apple juice concentration, the sp. Pyruvate decarboxylase activity (v) is increased, and then it is decreased as shown in Figure 7. For Michaelis-Menten enzyme kinetic model, the sp. Pyruvate decarboxylase activity (v) is determined as 2745 min-1 (upto linear portion of Figure 7) and maximum sp. Pyruvate decarboxylase activity (vmax ) is calculated as 5490 min-1 for ethanol fermentation of cashew apple juice respectively. The kinetic parameter, Km , (Km = S) is determined as 160.5 g/L (upto linear portion of Figure 7) for cashew apple juice fermentation in CSBR using S. cerevisiae.
The kinetics of aerobic bioethanol fermentation in CSBR using yeast S. cerevisiae was analyzed. The first order rate equation is shown by,
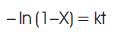
Where X : the conversion of cashew apple juice into alcohol at fermentation time (t).
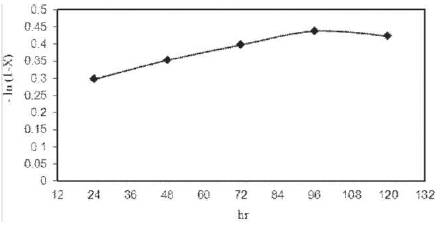
Figure 8. Rate kinetics model of ethanol fermentation
As shown in Figure 8, a straight line was obtained by plotting fermentation time (t) against [– ln (1–X)]. The rate constant (k) was measured from slope of the straight line. The rate constant (k) was found to be 0.0166 hr−1 for ethanol fermentation in CSBR using yeast S. cerevisiae. Kinetics of ethanol fermentation from cashew apple juice shows that it is well agreeable with the first order rate kinetics.
Fermentation of bioethanol from cashew apple juice with yeast S. cerevisiae in CSBR was found to be an effective biofuel production process. The optimum parameters for bioethanol fermentation are: time, 96 h; pH, 5.0; temperature(55ºC); stirring speed(35 rpm); cashew apple juice concentration(400 g/L). Maximum yield of ethanol was found as 0.493 g/g of cashew apple juice at optimum bioprocess parameters. The sp. yeast growth rate (µ) was 7.05 s−1 and maximum sp. yeast growth rate (µmax) was 14.10 s−1 using Monod model respectively. The max results suggested that various parameters drastically influenced the fermentation of ethanol by S. cerevisiae to a large extent. The studies could establish the optimized bioprocess parameters for maximum and effective utilization of cashew apple juice for ethanol fermentation for future biofuel. The yeast can grow reasonably well in cashew apple juice environment in CSBR that was evidenced by Monod Growth Model. An important case of decrease of yeast growth is that of product ethanol concentration and substrate. The utilization pattern of substrate cashew apple juice is significantly influenced by the adaptation characteristics of yeast culture. Adaptation of yeast significantly affects the sp. growth rate, and overall fermentation of bioethanol. The repression and depression processes allow S. cerevisiae to regulate their enzyme (Pyruvate decarboxylase) content in direct response to the environment. Aerobic bioethanol fermentation of cashew apple juice in CSBR followed the first order rate kinetics with rate constant (k) and was 0.0166 hr−1.