
Table 1. Nominal chemical composition (wt%) of the substrate materials along with Application
In the present study cold spraying was used to deposit NiCoCrAlY coating on Fe-based Superfer 800H superalloy and coating was successfully deposited with cold spray process. Sprayed coating was tested for microhardness, thickness and microstructure. The hot corrosion behavior of the bare and coated superalloy is evaluated in Na2SO4-10% NaCl environment at 900°C under cyclic conditions. The microstructural characteristics of the corrosion products were investigated with XRD(X-ray Diffraction), SEM(Scanning Electron Microscopy), EDAX (Energy-Dispersive Spectroscopy) and Elemental X-Ray Mapping. The cold sprayed NiCoCrAlY coating perform better as compared to bare superalloy against hot corrosion degradation. Dense coating structure obtained by the cold spray technique and protective oxide layer on the top are responsible for better performance of the coating.
Hot corrosion phenomenon is very important because of the failure of components such as boiler, internal combustion engine, aircraft, gas turbines and waste incinerator. The hot corrosion is accelerated attack in the presence of salt contaminants such as Na2SO4, NaCl, and V2O5 which combine and form molten deposits that damage the protective surface oxides layer (Edris et al., 1997). Metals and alloys sometimes experience accelerated oxidation when their surfaces are covered by a thin film of fused salt in an oxidizing atmosphere at elevated temperatures. This mode of attack is called hot corrosion, where a porous nonprotective oxide scale is formed at the surface and sulfides in the substrate (Rapp & Zhang, 1994;Rapp, 1986). The phenomenon of hot corrosion was first documented in 1940s in connection with the failure of fireside boiler tubes in coal-fired steam generating plants.
Although, superalloys have been developed for high temperature applications, the superalloy cannot fulfill the requirements of high-temperature strength along with the high-temperature erosion–corrosion resistance (Kamala et al., 2008). Consequently, we require a composite system of a base material which will provide the necessary mechanical strength along with a surface layer for the high-temperature erosion–corrosion protection. Thermal spray coating is one of the key solution for this problem. Thermal spray coatings had been studied by a number of researchers (Sidhu & Prakash, 2006; Sidhu et al., 2005; Goyal et al., 2012; Patnalk & Immarigeon,1989; Goyal et al., 2012; Bala et al., 2010; Richer et al., 2010) for high temperature application and they establish that use of coatings increase considerable life of materials.
In the thermal spray process like plasma, arc and flame spraying the velocity of spraying particle is low and temperature high. In High-Velocity Oxy-Fuel (HVOF), velocity of spraying particle is too high, but partially melting of particles takes place during flight time. However, in cold spraying process the kinetic energy of the particles is higher and temperature is lower as compared to other thermal spray process, so it helps these particles to plastically deform with impact and form splats, which bond together to produce coatings and thereby avoids or minimizes many deleterious shortcomings of traditional thermal spray methods such as high-temperature oxidation, evaporation, melting, crystallization, residual stresses and gas release. In this process, powder particles are accelerated by the supersonic gas jet at a temperature that is always lower than the melting point of the material, resulting in coating formation from particles in the solid state and hence no melting and solidification process is experienced by the powders like in traditional thermal spray process (Singh et al., 2012) Detail of cold spray development and its bonding mechanism was already discussed by authors (Singh et al., 2013). The sulfate-chloride based molten deposits are found to be highly aggressive in nature and are the major causes of failure of components of power plants, gas turbines working especially in marine based environment. MCrAlY (M=Ni,Co or Fe) corrosion resistant coatings were successfully applied by different coating methods such as Physical Vapor Deposition (PVD), Chemical Vapor Deposition (CVD), plasma spray, arc spray and flame spray and HVOF and tested for application on hot section of turbines, boilers, incinerators and petro chemical industries (Gurrappa, 2000; Gao et al., 1999, Bala, et al., 2010;Matsubara, 2007).
It is found from the literature that little work has been done on cold spray coating in Na2SO4-NaCl environment at high temperature and hence this technique is used to develop high temperature resistant NiCoCrAlY coating on Fe-based superalloy. The bare and coated superalloy is tested under hot corrosion environment of Na2SO4-10%NaCl based molten salt environment at 900oC.
The Midhani grade Fe based Superfer 800H superalloy substrate material provided by Mishra Dhatu Nigam Limited, Hyderabad (India) in the rolled sheet form is used in the present study. The composition along with some application of superalloy is provided in Table 1.
Commercially available Ni-22Co-18Cr-12Al-1Y powder (commercially name Carpenter Micromalt (CCW)) with particle size range 5-20 µm was used as feedstock material for coatings. The SEM micrograph of NiCoCrAlY powder used for coating is shown in Figure 1. The particle shape of powder is mixed, spherical and oval. The EDX analysis shows the presence of Ni (46%wt.),Co (24%wt.), Cr (20%wt.), Al (9%wt.) and Y(1%wt.) which is close to designated composition.

Figure 1. SEM micrograph of NiCoCrAlY powder
The bare superalloy specimens were cut from the sheet with dimension of 20x15x3mm. Polishing of specimen was done using SiC emery papers 100, 220, 400, 600 grit size. Then specimens were grit blasted with alumina powders (grit 45) before development of the coatings by Cold spray process.
Cold spray system was used for depositing the NiCoCrAlY coatings. Coatings were developed at ASB industries, Inc., barbeton Ohio with Kinetics 3000 from CGT technologies, Germany using Helium as process gas. Standard spray parameters, as mentioned in Table 2 have been used while coating the specimens. All the process parameters were kept constant throughout the coating process. After polishing the sample, the thickness has been measured from the Back-Scattered Electron Images (BSEI) taken along the cross section of the mounted samples as shown in Figure 2.

Table 2. Standard spray parameters

Figure 2. BSEI showing cross-section of cold spray coated NiCOCrAlY on Superfer 800H
The porosity of as-sprayed coating was measured with an image analyser using Dewinter Material Plus 1.01 software based on ASTM B276. The surface roughness of cold sprayed surface was measured with the help of surface roughness tester made (Jpan) mitutoyo model SJ201M. For surface analysis the coated samples were polished using SiC emery papers 220, 400, 600 grit,1/0,2/0,3/0,4/0 grades and subsequently on wheel polishing machine using 1 µm alumina powder suspension, then samples were subjected to SEM/EDAX analysis to characterise the surface of the coatings. A Scanning Electron Microscope [JEOL (JSM-6610LV)] resolution in high vacuum mode-3nm,magnification up to 300000 with EDAX attachment (Oxford: Model- INCA x-act 51-ADD0013, England) was used for SEM/EDAX analysis. XRD analysis was carried out with Bruker AXS D-8 Advance Diffractometer (Germany) with CuKa radiation and nickel filter at 20mA under a voltage of 35kV. For cross-sectional analysis the samples were sectioned and mounted in epoxy. The mounted specimens were prepared by polishing, using SiC emery papers of 220, 400, 600 grit and subsequently 1/0, 2/0, 3/0, and 4/0 grades. Fine polishing was carried out using a 0.3 µm diamond paste. Then the characterisation of specimen along the cross-section was carried out using Digital Micro Vickers Hardness tester and SEM/BSEI. Micro hardness along the cross-section of the coating was measured using a microhardness tester (Wilson, 402 MVD) under an indentation load of 15 grams with a diamond indenter.
Hot corrosion studies were carried out in silicon carbide tube furnace at 900°C with temprature variation of ±5°C.The studies were conducted for 50 cycles in molten salt environment of Na2SO4 -10%NaCl . Each cycle was consisted of 1h heating at 900°C followed by 20min cooling at room temperature. The samples were subjected to mirror polishing before the corrosion run. Thereafter, a coating of Na2SO4 -10%NaCl salt was applied on the pre heated samples at 250°C with the help of a camel hair brush. The coating thickness of Na2SO4 -10%NaCl salt was kept as uniform with 3 to 5 mg/cm2. Then the samples were dried in oven at 110°C temperature for 3–4h to remove the moisture. The weight of samples was measured after each cycle of study. The samples were visually examined at the end of each cycle for any change in the colour, lustre, adherence of scale to the substrate and spalling tendency. After 50 cycles of studies, the corrosion product on the surface was studied with XRD and SEM/EDAX analysis. For the cross-section study the corroded samples were prepared in similar way as discussed above for as-sprayed coating. Then prepared specimens were analysed by SEM/EDX and X-Ray mapping.
The coating thickness was measured from the BSE image as shown in Figure 2. The Average thickness of the as-sprayed coating on the substrate superalloy was found to be 246 µm. The porosity of cold sprayed NiCoCrAlY was found to be in the range of 0.8-0.9. The average surface roughness of cold sprayed sample was measured to be 6.22µm.
The microhardness of coating on substrate superalloy has been measured along the cross-section and data is compiled in Figure 3. The microhardness values of the substrate superalloy and coating is found to be in the range of 260-380 Hv and 582-640 Hv respectively. Although uniform hardness was observed across the cross-section of coating except small variations in hardness. This variation of harness values may be due to the presence of porosity and voids.
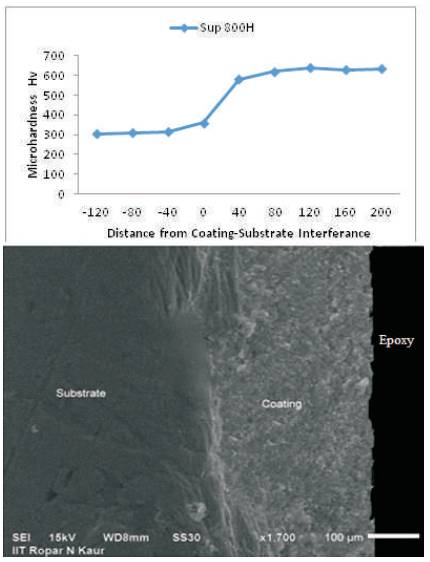
Figure 3. Microhardness Vs Distance from coating-substrate interference for cold sprayed NiCoCrAlY coating on the superfer 800H superalloys
Surface SEM micrographs of NiCoCrAlY cold spray coated superfer 800H is shown in Figure 4. The SEM micrograph indicates irregular rock like structure. In cold spray process bonding is due to high velocity and particles do not melt in-flight, so no oxidation of particles in-flight takes place. Also it is found from the EDAX, there is only 1% oxygen was found. So it shows negligible amount of oxides to be formed. Dense structure of the coatings may be attributed to the high impingement velocity.
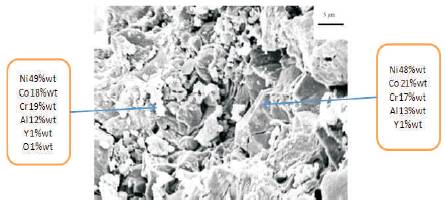
Figure 4. SEM/EDAX micrograph of NiCoCrAlY coated surface of superfer 800H
The macrograph of hot corroded bare Superfer 800H superalloy after 50h of cyclic exposure in molten salt Na2SO4-10%NaCl environment at 9000C is shown in Figure 5a. After first cycle of exposure the color of exposed bare Superfer 800H found to be light black which changes to dark black during subsequent cycles of exposure. Also some greenish tinges were observed after 6th cycle onwards. The spalling of the scale was found to be started from the third cycle onwards in the case of bare Superfer 800H which intensified as the period of hot corrosion study progressed, and removal of the top scale was observed after 17th cycle of the study.
The macrograph for the hot corroded surfaces of cold sprayed NiCoCrAlY coated Superfer 800H superalloy after 50 cycles of study is shown in Figure 5b. The surface color of the coated specimen turned to dark grey after first cycle. Some tinges of greenish color appears after 5th cycles of study. The bluish and greenish spots were found in the dark grey background after 17th cycle onwards. No spalling of scale was observed during the hot corrosion study.

Figure 5. Macrograph of the surface exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 9000C a) Bare superfer 800H b) NiCoCrAlY coated
The thermogravimetric data of hot corroded bare and cold sprayed NiCoCrAlY coated Superfer 800H during 50h of cyclic study in the given environment is shown in Figure 6. The bare Superfer 800H superalloy shows a much higher weight gain as compared to NiCoCrAlY coated superalloy in the given environment. The cumulative weight gain/ unit area after 50 cycles of exposure of the specimens is shown in Figure 7. The weight gained by NiCoCrAlY is approximately 1/4th of the weight gained by bare superalloy, and indicates the protective nature of the coating. The weight change/area squared versus the number of cycles for NiCoCrAlY coating have shown the parabolic behaviour during the cyclic study (Figure 8), however, the plots for bare superalloy shows significant deviation from the parabolic behavior. The parabolic rate constant kp was calculated by a linear least-square algorithm function in the form of (∆W/A)2 = kp∙ t, where ∆W/A is the weight gain per unit surface area (mg/cm2), t is the hot corrosion time in seconds. The parabolic rate constant, kp (x10-10 g2cm-4s-1) for bare Superfer 800H and NiCoCrAlY coated superalloy calculated on the basis of 50 cycles data is found to be 128.541 and 5.972, respectively, indicating the better hot corrosion protection provided by the cold spray coating to the superalloy used in this study.
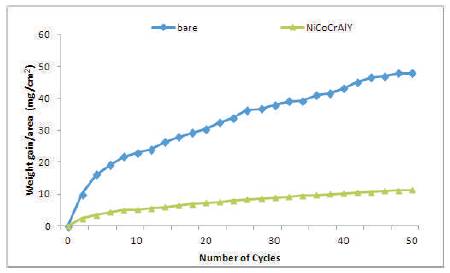
Figure 6. Weight gain vs. number of cycles plot for the coated and bare Superfer 800H exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 900oC
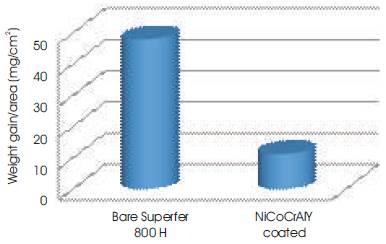
Figure 7. Bar charts showing cumulative weight gain per unit area for the coated and bare Superfer 800H exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 9000C
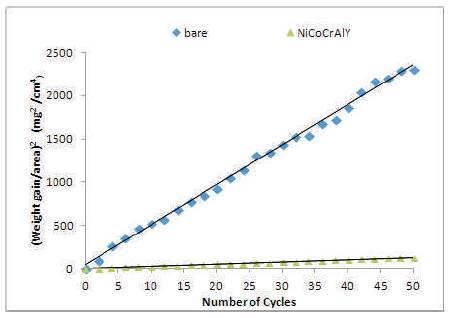
Figure 8. (Weight gain/area)2 vs. number of cycles plot for the coated and bare superalloy Superfer 800H exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 900oC
The diffraction patterns for the NiCoCrAlY coated Superfer 800H after hot corrosion for 50 cycles at 900oC indicates the Cr2O3 and CoO as main phase along with Al2O3, NiCr2O4 and CoCr2O4 (Figure 9). Whereas Fe2O3, NiO, were identified as the main phase along with Cr2O3, NiFe2O4, CrS, and FeS in the surface of the bare superalloy after the exposure in the given environment.
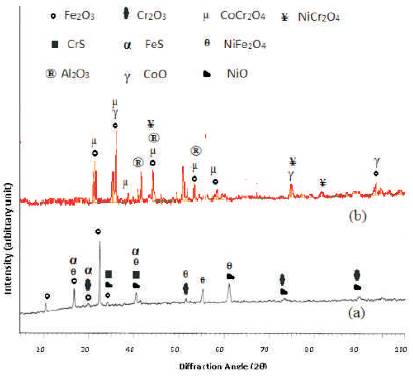
Figure 9. X-Ray diffraction profiles for bare and coated superfer 800H exposed to cyclic hot corrosion in Na2SO4-10%NaCl at 9000C for 50 cycles. (a) bare superfer 800H (b) NiCoCrAlY coated
SEM micrographs showing surface morphologies along with EDAX analysis of the corroded bare and NiCoCrAlY coated Superfer 800H have been shown in Figure 10. The scale of bare Superfer 800H contains Fe as the predominant phase along with significant amounts of Ni, Cr and O in Figure 10a which indicate the possibility of Fe2O3, Cr2O3 and NiO. The cracks produced in the scale and spalled region are clearly visible where more amount of Fe2O3 and lesser Cr2O3 is found. The scale formed on the hot corroded surface of the NiCoCrAlY coating deposited on Superfer 800H has almost consistent and continuous (Figure10b). The scale has granular appearance. The scale formed has Cr as a principal phase along with some percentage of Co and Ni phases. Sufficient amount of Oxygen is also present to form oxides. So surface contains Cr2O3 as major phase along with, NiO, and CoO. A small amount of Y2O3 is also present in the scale. The small amount of oxides of Ti, Si are found in the surface oxide layers indicate that these elements have diffused from the substrate through the coating to the uppermost part of the scale during hot corrosion cycles in molten salt environment. The presence of Cl, Na and S indicates the presence of molten salt elements even after 50 cycles of hot corrosion.
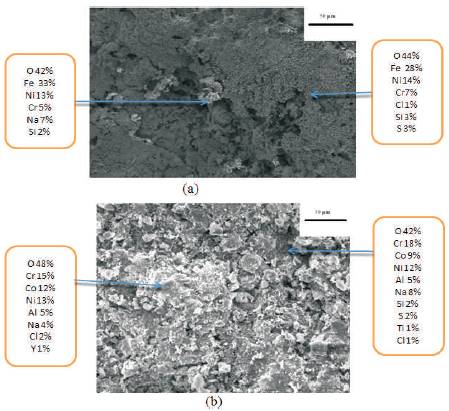
Figure 10. SEM/EDAX showing surface exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 9000C (a) Bare superfer 800H (b) NiCoCrAlY coated
SEM (BSE) image and elemental variation for the hot corroded cross-section of bare and NiCoCrAlY coated superfer 800H after 50 cycles of exposure are shown in Figure 11. SEM/EDAX analysis of bare 800H superalloy at point1 shows the presence of oxide scale rich in Fe and Ni at the top, with some amount of Cr, Cl and S as shown in Figure 11a. The Fe2O3 is present throughout the cross-section. The cracks are clearly visible on the scale. At the point 2 Ni increase and Fe decrease as compare to point 1 which shows the high concentration of NiO at point 2. Oxygen has diffused into substrate up to point 5.
SEM image shows a thin white colour layer at the top for NiCoCrAlY coated superfer 800H (Figure 11b). The EDAX analysis at the point 1 on the thin white layer indicates oxides rich in Cr and Co along with some oxides of Ni, Y and Mn. Therefore this thin layer might be Cr2O3 and CoO rich scale. EDAX analysis shows that the oxygen is penetrated upto point 5. The presence of Mn and Ti in the scale indicates the diffusion of these elements from substrate to scale through the pores and splat boundaries present in the coating.
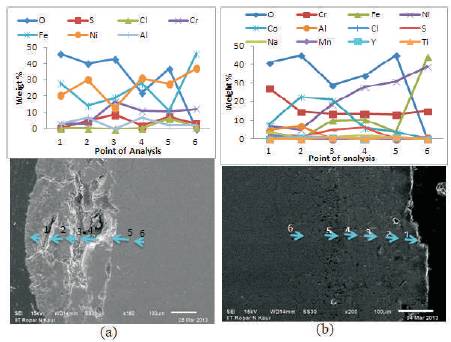
Figure 11. Cross-sectional EDAX of NiCoCrAlY coated and bare superfer 800H exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 9000C. a) bare Superalloy b) coated superalloy
The elemental X-ray mappings at the cross-section of bare and NiCoCrAlY coated superfer 800H after cyclic hot corrosion in Na2SO4-10%NaCl environment at 900oC for 50 cycles are shown in Figure 12 and Figure 13 respectively. The BSE image of bare superfer 800H after 50 cycles of hot corrosion in the given molten salt environment show different layers (Figure 12). Element X-ray mapping reveals that the top layer of the scale has high concentration of Fe indicating Fe2O3 scale at the top. The second layer just below the upper layer contains the Cr as major phase along with small quantity of Al and Si. Therefore beneath top layer the scale is found to be rich in Cr2O3. The elements Mn, Na and Cl show their presence randomly throughout the cross-section.
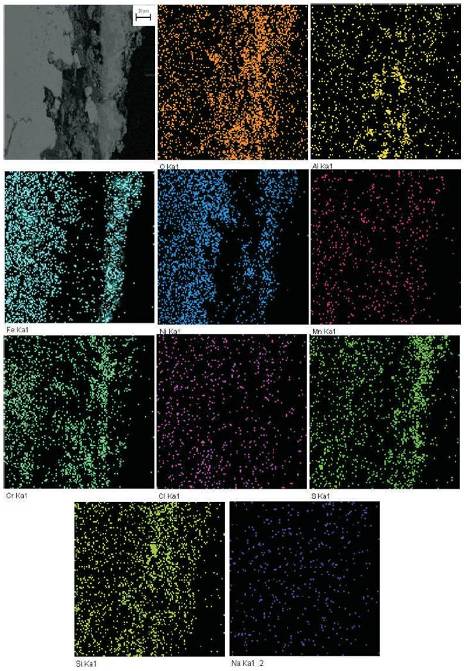
Figure 12. BSEI and X-ray mapping of the Bare Superfer 800H exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 9000C
BSE image shows a dense continuous and adherent scale at the top (Figure 13). Element mapping analysis across the cross-section of NiCoCrAlY coated superalloy shows the presence of Cr rich scale on the top with some amount of Co after 50h of exposure in the given environment. Beneath layer mainly consist of Cr, Co, Al and Ni. There is also a presence of Ti, Fe and Mn. These elements may be diffused from the substrate to scale during hot corrosion.
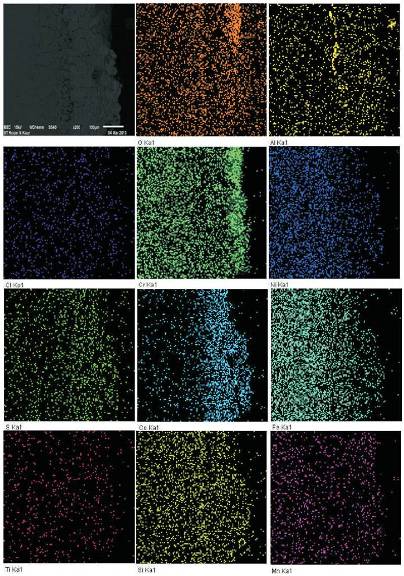
Figure 13. BSEI and X-ray mapping of the NiCoCrAlY coated Superfer 800H exposed to hot corrosion for 50 cycles in Na2SO4-10%NaCl environment at 9000C
The NiCoCrAlY coating was successfully deposited on superfer 800H with cold spray process. Hardness is very important property of coating especially for the application of coating in corrosion-erosion environment. The microhardness value of the substrate near substrate-coating interference is found to be higher which can be attributed to strain hardening due to the high impingement velocity of the spraying particle as proposed by Bala et al., 2011. The microhardness of coating is very high as compared to substrate. The high microhardness of the coating can be attributed to the cold working effect on the feedstock powder (Jodoin et al., 2006). The high micro hardness partially may be attributed to the low porosity of the coating.
The porosity of the coating is very important factor for corrosion resistance performance of the coating. The porosity for good corrosion resistance coating should be as low as possible. The porosity of the coating was found to be less than 1% in the present study. The results are in good agreement with the finding of Richer (Richer et al., 2010). As the porosity is quite low, so coating should have good resistance against hot corrosion.
The coating thickness has significant role for life of material against hot corrosion. Gurrappa, (2000) reported that life time increases with increase in coating thickness and further found maximum life with 300 µm coating thickness. In the present study average coating thickness was observed to be 246 µm which is sufficient to protect the substrate from hot corrosion attack.
The bare Superfer 800H superalloy under study has shown more weight gain as compared to the coated superalloy in the molten salt environment of Na2SO4-10%NaCl. The weight gain of the bare superalloy was observed throughout the study, but more weight gain was found during initial cycles of study. This higher weight gain during initial might be attributed to the rapid oxidation of the surface elements to form oxide scale. The high weight gain during initial cycles of hot corrosion was also reported by Tiwari et al., 1997; Singh et al., 2005; Sidhu et al., 2006. The weight gain plot shows the deviation from the parabolic rate law due to the spalling and sputtering of the oxide scale.
The higher weight gain in case of bare superfer 800H may be attributed to the formation of Fe rich oxide scale which is less protective in nature as compared to the oxides of Ni or Cr resulting in more spalling and sputtering. The Cr2O3 formed in the top layer scale is very small and is unable to provide protection against the hot corrosion.
The cold spray coating used in this study on the superalloy shows the highly protective nature against the hot corrosion degradation in the given molten salt environment. The weight gain by cold sprayed NiCoCrAlY coating is quite less than the bare superalloy understudy. Higher weight gain was found during initial cycles of study and it has become gradual with the progress of study in case of coated superalloy. The higher weight gain during the initial cycles of study might be attributed to the rapid formation of protective oxides on the surface, pours and at splat boundaries. These protective oxides plug the pours and splat boundaries which slow down the further oxidation and flow the parabolic law of oxidation. The overall weight gain by coated Superfer 800H is 11.242 mg/cm2 which is quite less than weight gain of 47.993mg/cm2 by bare Superfer 800H in the molten salt environment after 50 cycles. Hence coating is able to reduce the weight gain of the superalloy by more than 76% in the given molten salt environment. The NiCoCrAlY coated superalloy also follows the parabolic rate law of oxidation with some little deviations. These deviations might be attributed to the formation and rapid growth of inhomogeneous oxides, and their dissolution due to the fluxing action of the molten salt.
The formation of dense structure of the coating has also contributed in developing hot corrosion resistance at higher temperature, since corrosive species mostly propagate along the splat boundaries and through the pores and voids. Due to dense and flat splat structure of the coatings, the distance from the coating surface to coating-substrate interface along splat boundaries is highly increased which enables the coatings to develop resistance against hot corrosion.
BSE image in Figure 13 indicates that the partially oxidised coating has successfully maintained its integrity on the substrate superalloys under study. Moreover the top scale has high concentration of Cr, and Co along with Al as shown in EDAX analysis Figure 11. The oxides phases of Cr, Co and Al are also detected with XRD. The presence of Co, Cr and Al at the top layer can be seen from the elemental mapping reported in Figure 12. Hence, these oxides of chromium, aluminum and Cobalt act like a diffusion barrier to the corrosion species and prevent the further corrosion. The results are consistent with findings of Gurrappa (2000). The author studied the hot corrosion behaviour of different sputtered MCrAlY coatings in molten salt environment of Na2SO4-10%NaCl and found that the NiCoCrAlY coating has provided highest corrosion resisting among all the coatings. Further Wang et al. (2004) reported the formation of protective Cr2O3 as well as non-protective NiO, NiCr2O4 or sulfates during the hot corrosion of NiCoCrAlY coatings in molten salt of Na2SO4 -25%K2SO4 at 900oC. The better hot corrosion resistance of coatings can be partially attributed to the presence of 1% Yttrium in coatings. Hanyi (1990) studied the effect of addition of Yttrium on hot corrosion of CoCrAl coatings in Na2SO4-25% NaCl environment at 900oC. They pointed out that the addition of 0.5% of yttrium in the sputtered Co-30Cr-6AI-0.5Y coating can remarkably improve its resistance to hot corrosion in molten sulphate salts with or without NaCI.
The XRD patterns show the formation of the oxides of chromium and cobalt, with some spinels of cobalt-chromium and nickel-chromium and the formation of these phases are confirmed by the EDAX and elemental mapping analysis. The formation of these oxides and spinels provides protection against the hot corrosive environment at high temperature. The formation of spinel CoCr2O4 blocks the diffusion activities through the cobalt oxide (CoO) which further decrease the formation of this oxide as reported by Luthra (1985). It is further reported that increase in the growth of CoCr2O4 and Cr2O3, in competition with CoO and Co3O4 formation, decreases the corrosion rate of alloys. Therefore NiCoCrAlY coating on superfer 800H can be used in components of power plants, gas turbines working in sulfate-chloride environment and can enhance the life of components.
The first author thankfully acknowledges the Punjab Technical University, Kapurthala, Punjab, India for carrying out this R&D work.