
Figure 1. Microscopic view of Spirulina Sp
The main objective of this paper is to develop and standardize the production method of Arthrospira platensis (Spirulina sp.) and to transform the algal oil to biodiesel by transesterification. The development of technique for the production of algal oil involves following three steps. The primary step is the preparation of BG 11 medium for the growth of micro algae. The second step involves the harvesting and extraction of the algal biomass using hexane and ether solvents and the reaction is maintained at 60oc for 24 hrs. The final step is the transesterification of the extracted algal oil using Sodium hydroxide (NaOH) as alkaline catalyst and methanol at 75oc for 48 hrs. The fatty acid methyl ester thus produced is separated from glycerin using an inverted separator. The physio-chemical properties were found to be within ASTM standards.
Now-a-days, the world is facing a stringent problem for transportation (i.e.) in one aspect, the depleting fossil fuel and in the other hand, the price hike of petrol-diesel [1][5]. This gives a way for a suitable alternative renewable energy to face the crisis in the future. There are various renewable energy with respect to vegetable oil like cotton seed oil, rape seed oil, jatropa oil, mahua oil and many more [21]. All the listed vegetable oil needs more quantity of land and time to obtain the vegetable oil which is the major drawback. Here a major role is played by micro algae like Chlorella Sp, Spirulina Sp, Spirogyra Sp, Euglena Sp, Scenedesmus Sp, and other [2][6] for the production of Biofuel. In this study the ability of Spirulina sp in the production of Biofuel is characterized. The microalgae not only produces biodiesel but also helps in carbon-dioxide fixation [10] in the atmosphere due to photosynthetic reactions, thereby helps in global warming. The microalgae also find application in treatment of waste water in the literature [12].
Alkali-catalysis transesterification gives high level of triglyceride conversion into mono esters in short period and widely used in several countries. Now-a-days, enzymatic transesterification also gain more importance because the recovery of glycerol which is the by-product of transesterification is easier and purification of fatty acid methyl ester is simpler [24]. Transesterification process for cotton seed oil, hazelnut kernel, poppy seed, rapeseed, safflower seed and sunflower seed oil was carried out using super critical methanol without any catalyst and investigation like molar ratio of alcohol to vegetable oil and reaction temperature was carried out which resulted in increase of viscosity for biodiesel [25].
Biofuel is also extracted from Chlorella sp. using BG11 media composition. The algal oil is converted into biodiesel using acid-catalyzed transesterification (In situ process) in which the conversion efficiency is estimated using the specific gravity of biodiesel. It is identified that by increasing the moisture content of the Microalgal biomass, a strong negative effect was seen on the equilibrium of FAME yield [26]. Lipid extraction was carried out from dry biomass of Chlorella sp. primarily using chloroform and methanol in ratio 2:1 followed by methanol, ethanol, chloroform and hexane separately. The extraction of lipid using methanol was smaller but its conversion efficiency to FAME's was higher from lipids [23].
The cell wall membrane of microalgae comprises of cellulose, chitin, murein, protein, silica and calcium carbonate. The wall membranes can be ruptured using the milling, high pressure homogenization, ultra sonicator, centrifugation, microwave method and solvent extraction method to extract algal oil [27]. The growth of any algal system mainly depends on the availability of light factor and plays a major role in increasing the yield of algal biomass, in turn leads to higher fatty acid content in the biodiesel production [8][10]. The transesterification process yields high quantity ester of fatty acid [14] content due to chemical reaction between crude algal oil and methanol in the presence of alkali catalyst like sodium hydroxide or potassium hydroxide. 1% of sulphuric acid is added to the crude biodiesel before the esertification reaction to prevent the formation of soap due FFA's [16] [21].
The culture sample is obtained from the biological farm in Chennai. The characteristic study and the morphological study are carried out with the microscopic view of Spirulina Sp (Figure 1). The BG11 medium is prepared for the growth of microalgae in the photo bioreactor in an aseptic condition. The medium was sterilized at 100oc for 20 min before the growth phase of microalgae and continuous light is provided for enhanced growth [2].

Figure 1. Microscopic view of Spirulina Sp
Nutrients like nitrogen, phosphorous and potassium are important for plant growth and are essential parts of fertilizer. Silica and iron, as well as several trace elements, may also be considered important marine nutrients as the lack of one can limit the growth and productivity in an area [22]. The detailed media composition is given below
Media Composition for Spirulina Sp. (BG11 Medium)
To Fog's Medium add 0.2% Kno3
Fog's Medium
MgSO4.7H2O - 0.2 g
K2HPO4 - 0.2 g
*Micronutrient - 1.0 ml
Solution
CaCL2. H2O - 0.1 g
*Fe-EDTA solution - 5.0 ml
Distilled water - 1.0 L
Adjust pH to 7.5
*Micronutrient Solution
H3BO3 - 286.0 mg
MnCL2.4H2O - 181.0 mg
ZnSO4.7H2O - 22.0 mg
Na2MoO4.2H2O - 39.0 mg
CuSO4.5H2O - 8.0 mg
Distilled water - 100.0 ml
*Fe- EDTA solution
In hot water dissolve 745.0 mg of Na2EDTA and then add 557.0 mg of FeSO4.7H2O. Boil the solution for few minutes and make the volume to 100.0 ml. After 120 hrs of the growth phase, the algal biomass is harvested and dried in the incubator at 50oC for 3 hrs to remove excess moisture [6]. Solvent extraction method is employed for separation of Algal oil from biomass. N-Hexane and Petroleum ether solution (30 ml and 30 ml respectively) were mixed with the dried ground algae to extract oil. Then the mixture was kept for 48 hours for settling. The biomass was collected after filtration and weighted. The algal oil separated from the biomass by applying pressure on the colloidal mixture with the help of a centrifuge. The extracted oil was evaporated in vacuum to release n-hexane and Petroleum ether solutions using rotary evaporator [9] [7].
The transesterification reaction is performed using 20% methanol and 5% of Sodium hydroxide as a catalyst. The mixture is kept in a rotary agitator at 200 rpm for 16 hrs and then allowed to settle down for a period of 48 hrs. The glycerin formation confirms the transesterification reaction and it is removed using a separator until 95% of FAME's is derived.
The obtained AOME is tested for various properties like density, Specific gravity, kinematic viscosity, water content, ash content, calorific value and cetane number [4], and they were found to fall within the ASTM standards (Table 1).
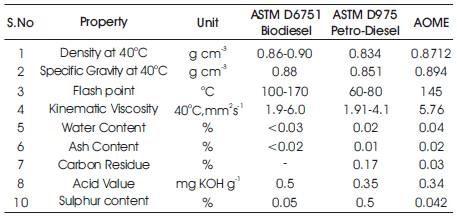
Table 1. Properties of AOME in comparison with ASTM D6751 and ASTM D975
The extracted algal oil methyl ester is characterized for the physical and chemical properties like density, specific gravity, kinematic viscosity, flash point, water content, carbon residue, acid value and sulphur content. The specific gravity at 40oC for AOME was found to be 0.894 g cm-3 which lies closely in the limits of ASTM D975 for petrodiesel as 0.834 and ASTM D6751 for biodiesel as 0.880. The density at 40oC being one of the most important factor is found to be 0.8712 g cm-3 in comparison with biodiesel (0.86-0.90) and petro-diesel (0.834) under ASTM standards [4][19].
The kinematic viscosity is another important property of the fuel because it offers internal resistance to flow of fuel and it also affects the fuel injection system of the engine. The viscosity at 40oC is determined as 5.76 mm2 s-1 which is comparable with ASTM D 6751 and ASTM D975. The flash point plays a vital role in fuel handling and storage and found to be 145oC. On comparision, it lies above ASTM D975 of petro-diesel. This reveals that AOME is more safer than petro-diesel in handling and storage. It also falls within limits when compared with ASTM D6751.
Even though the presence of water molecule in the fuel in undesirable, there are some traces of it. The water content in AOME was found to be 0.04% and lies slightly on the higher side on comparison with ASTM D975 and ASTM D6751. The ash content denotes the content of metal in the fuel. It is to be as low as possible in order to prevent plugging of injector tip[7] and deposition occurence during combustion in the walls. The ash content is found to be 0.02% in AOME which is comparitively higher in petrodieseland lower in biodiesel. Carbon residue indicates the presence of carbon particle [17] in the chamber after combustion and it should be lower. In AOME 0.03% of carbon residue was found when compared to 0.17% in ASTM D975. The presence of free fatty acid is indicated by the acid value. In AOME, the acid value is found as 0.34 mg KOH g-1 which is comparitively lower than ASTM D975 and ASTM D6751. The content of sulphur in AOME is determined as 0.042% which is significantly lower than petro-diesel and biodiesel.
During the transesterification reaction at room temperature, methanol is added to the algal oil. Primarily the rate of reaction was very high at 50% [9] during the first hour. But after, the reaction was very much reduced due to the formation of water molecule as a result of esterification and the reaction rate was less than 20%. The water formation interupts the formation of methyl ester during the reaction. Further the reaction ended with no significant changes [13].
Later the similar reaction was carried out at 50oC. The transesterification rate was found to be 78% initially[13]. The reaction rate is continued by adding more quantity of methanol to the algal oil and maintaining the reaction at 50oC and a molar ratio of 6:1[11]. It is also noted that the presence of FFA's in the algal oil also reduces the ester formation (Figure 2). The ratio of FFA's is reduced by adding by mixing 1% conc sulphuric acid to the algal oi which reduces the FFA's content from 12% to 2% [20].
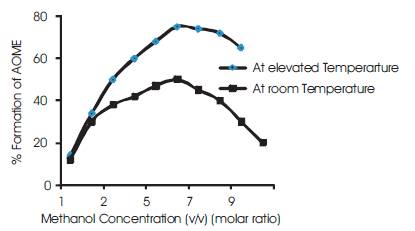
Figure 2. The effect of AOME formation with methanol concentration at room and elevated temperature
During the transesterification process 1% of Sodium hydroxide is added to the algal oil with methanol at 50oC [13]. At this concentration, the esterification process produced a better yield (Figure 3) of FAME's but on further increase of the concentration of the catalyst resulted in reducing the yield of the ester by a formation of gelatin layer which prevent the reaction rate[15,18].
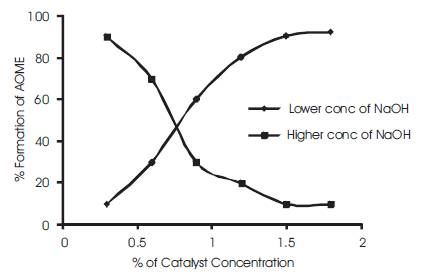
Figure 3. The effect of AOME formation with catalyst concenrtration at low concentration and high concentration levels
The BG11 medium is extremely suitable as the growth supplement for the micro algae for the higher yield of biomass. The biomass yield was found to be 85%. The seperation of algal oil from the biomass using hexane and ether gave a good yield of 90%. The free fatty acid content of the algal oil is reduced to 2% from 12% by adding sulphuric acid. Further the transesterification reaction followed with Sodium hydroxide and methanol yielded AOME about 95%. The FAME formation was found to higher at 50oC to 60oC with increased concentration of methanol. The derived AOME is tested for various physio-chemical properties like Kinematic viscosity, Density, Specific gravity, Sulphur content, Flash point Ash content and carbon residue and they are within the standards of ASTM.
| EDTA | - Ethylenediamine tetra acetic acid |
| AOME | - Algal oil methyl ester |
| FAME | - Fatty acid methyl ester |
| Bg11 | - Blue green medium |
| KOH | - Potassium Hydroxide |
The author would like to thank ITA Lab Services (an ISO certified organization),Chennai, India for testing the AOME samples.