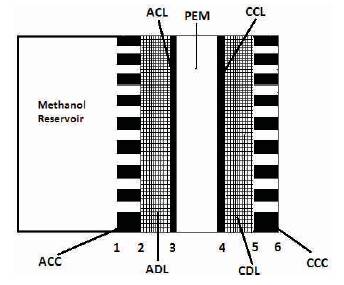
Figure 1. Schematic of passive DMFC
A Direct Methanol Fuel Cell (DMFC) directly converts the chemical energy stored in methanol to electricity. DMFCs have received considerable attention as a new power source for electric portable devices because of their high theoretical energy densities. In particular, passive DMFCs can provide a higher energy density then active one, since they do not need pumps for fuel feeding and blowers for air breathing. However, the actual energy density of the passive DMFCs under development is still far from that expected because of the methanol crossover (MCO) and the high overvoltage at the electrode. Due to the MCO, the passive DMFCs usually show the highest performance at low concentrations of methanol about 5M under passive conditions and that's why diluted methanol (3M-5M) is used in the fuel cartridge. Filling the fuel reservoir with a low-methanol concentration means that the specific energy of the fuel cell system is low, which not only leads to a short operation time for each fuel charge, but also results in a rapid decrease in the pre-set methanol concentration in the fuel reservoir. Hence, filling in the fuel reservoir with a high-methanol concentration is desired, as it increases the volumetric energy density and discharging time of the DMFC system. This paper focuses on the progress and current status of research in the passive DMFCs fed with concentrated methanol. The paper reviews more than 40 journal and conference papers in this area and will be very useful to the researchers working in this direction.
Fuel cell is a device that converts the chemical energy stored in a fuel directly into electrical energy and heat through the electrochemical reactions. Fuel cell systems have received much attention in recent years because of the increasing price of petroleum and its potential for wide range of applications. Fuel Cells are expected to be the next generation of power sources with the advantages of high efficiency and low pollution. Today, Fuel cells are extensively being studied because of its potential for a wide range of applications. (Faghri & Guo, 2008; Guo & Faghri, 2008; Chan et al, 2008; Kim et al, 2006).
Amongst the various types of fuel cells, the Direct Methanol Fuel Cells (DMFC) have attracted extensive interest from the research community and industry since its has the advantage of easy storage of liquid fuel, direct fuel feeding without reforming, low operating temperature and compact size (Cho & Kim, 2009). DMFCs can be categorized in active and passive types, according to fuel and oxidant supply modes (Park & Kim, 2011). Passive DMFC has the advantage of not having moving accessories e.g. pump and blower for fuel and oxidant supply or removal. That is why; these are more suitable for portable application and are considered as great alternative to rechargeable batteries (Xu & Faghri, 2010).
In particular, passive DMFCs can provide a higher energy density than active one, since they do not need pumps for fuel feeding and blowers for air breathing. However, the actual energy density of the passive DMFCs under development is still far from the expected because of the methanol crossover (MCO) and the high overvoltage at the electrode (Seo & Lee, 2010; Saarinen et al, 2007; Heinzel & Barragan, 1999; Du et al, 2007; Birry et al, 2009; Chen & Zhao, 2007). Due to the MCO, the passive DMFCs usually show the highest per formance at low concentrations of methanol about 5M under passive conditions and that's why diluted methanol (3M-5M) is used in the fuel cartridge. Filling the fuel reservoir with a low-methanol concentration means that the specific energy of the fuel cell system is low, which not only leads to a short operation time for each fuel charge, but also results in a rapid decrease in the pre-set methanol concentration in the fuel reservoir. Hence, filling in the fuel reservoir with a high-methanol concentration is desired, as it increases the volumetric energy density and discharging time of the DMFC system. This will help in competing with Lithium-ion batteries, as major application area of these are portable electronic devices e. g. laptop, mobile, iPod etc.
The purpose of this review is to summarize recent advances in the development of DMFCs operating with highly-concentrated ethanol solution and highlight future research directions. The remainder of this article is organized as follows: Section 1 gives a general description of DMFC systems with detailed mass transport mechanism. Necessity of using concentrated methanol in passive DMFC and ways to achieve that are elaborated in Section 2. Section 3 concentrates on the use of concentrated methanol fuel by inserting methanol barrier layer (MBL). Finally, a summary is given.
Construction of the passive DMFC is shown in Figure 1. Its components are fuel reservoir, Anode Current Collector (ACC), Anode Diffusion Layer (ADL), Anode Catalyst Layer (ACL), Polymer Electrolyte Membrane (PEM), CATHODE CATALYST Layer (CCL), Cathode Diffusion Layer (CDL), Cathode Current Collector (CCC).

Figure 1. Schematic of passive DMFC
The PEM conduct protons from the anode to the cathode, and also acts as an electronic insulator between the anode and the cathode. The Diffusion Layers (DL) provides support to the corresponding catalyst layer (CL), distributes reactants uniformly over the CLs and also conducts electrons to the current collectors. CLs are composed of catalysts and ionomer to provide triple phase boundaries for the methanol oxidation and oxygen reduction reactions.
Methanol and water are transported from the fuel reservoir to the ACL mainly by diffusion (concentration gradient exist between them). In the ACL, part of transported methanol is oxidized to generate protons, electrons & carbon di-oxide. The CO is transferred back to 2 the fuel reservoir due to combined effect of pressure difference and buoyancy force. The anode electrochemical reaction:
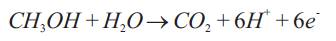
While, the remainder of methanol permeates through PEM, reaches at CCL where it gets oxidized. This makes adverse effect on cell voltage and accounted as “loss”. Oxygen from atmosphere reaches to CCL mainly by diffusion. At the CCL, part of the oxygen reacts with the protons that are conducted through the PEM and the electrons that come from the external circuit and form water as follows:
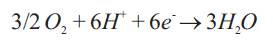
Remaining oxygen reacts with the permeated methanol and CO & water is produced. The part of water gets 2 evaporated and goes out of cell by diffusion. Remaining liquid water leaves the cell from cathode side due to combined effect of gravity and capillary force.
The adequate methanol concentration has to be managed at ACL. Increase in methanol concentration will lead to methanol crossover (MCO) whereas, decreased concentration result in increased concentration loss. To manage this level at ACL, 3 M – 5 M methanol solution is used in fuel reservoir (Liu & Zhao, 2005). But due to less methanol content in the solution, the system energy density of DMFC becomes low (Cai & Li, 2011). This leads to short operation time of the cell. Passive DMFCs are expected as potential alternative to rechargeable batteries. Hence, increasing the cell energy density is a prime requirement.
As shown in Figure 2, increased cell energy density can be achieved by using concentrated or pure methanol in the fuel reservoir. But, as discussed earlier this will increase the methanol concentration at ACL (more than adequate level) which finally results in increased MCO and cell voltage loss. So, encounter of MCO can facilitates the use of concentrated methanol fuel in the cell. Keeping this in mind researchers tried for concentrated methanol operation of passive DMFC by fallowing ways:
a. Development of low methanol crossover membranes (Jaafar et al, 2011; Lin et al, 2005; Yoon et al, 2009; Lee et al, 2008; Dai et al, 2008; Wei et al, 2012; Yang, 2008; Maiti et al, 2011; Tsai et al, 2007).
Extensive efforts have been taken in this direction. But it was observed that almost all new developed membranes suffers from side effect of decreased proton conductivity and increased cell internal resistance, hence degrading the cell performance.
b. Development of highly active anode catalysts (Li et al, 2012; Hiromi et al, 2011; Kakati et al, 2011; Yuan et al, 2011; Lin et al, 2011; Li et al, 2010; Zhu et al, 2010; Zhiani et al, 2010; Hirakawa et al, 2010). Pt–Ru has exhibited the best performance for the methanol oxidation reaction among all the catalysts currently available. But, its activity is still not sufficiently high to encounter the rate of methanol crossover.
c. Reduction of methanol crossover by improving Membrane Electrode Assembly (MEA) and cell design (Abdelkareem & Nakagawa, 2006; Tsujiguchia & Abdelkareem, 2010; Faghri & Xu, 2010; Pan, 2006; Wu et al, 2010; Abdelkareem & Nakagawa, 2007; Abdelkareem et al, 2007; Nakagawa et al, 2006) i. e. modifying design of anode diffusion layer or introducing an additional layer on methanol feed path, to reduce the methanol delivery rate (increasing the mass-transport resistance of methanol from the fuel reservoir to the anode catalyst layer). This is a relatively new approach and cheap, easy to implement and is able to produce effective results. In the next section this alternative is discussed in detail.
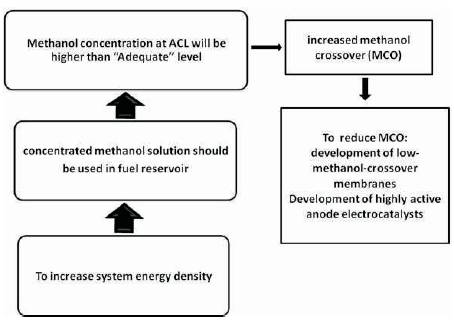
Figure 2. Increasing energy density of passive DMFC
Concentrated methanol feed operation of passive DMFC was achieved by inserting a methanol barrier layer (MBL) by several researchers. MBL was inserted between reservoir and ACL, which increases the methanol transport resistance. In this way, methanol concentration at ACL is maintained at 'adequate' level while, operating the cell with concentrated methanol fuel. Various materials, Porous carbon plate (Abdelkareem & Nakagawa, 2006; Tsujiguchia & Abdelkareem, 2010), porous PTFE plate (Faghri & Xu, 2010), Nafion membrane (Pan, 2006), micro fluidic-structured anode flow field (Wu et al, 2010) were used as MBL.
The effect of employing a porous carbon plate on the performance of a passive direct methanol fuel cell (DMFC) under closed circuit conditions was investigated by Abdelkareem and coworkers (2006; 2010) Figure 3. It was found that the porous carbon plate and a CO2 gas 2 layer that formed between the anode and the porous plate stably controlled the mass transfer of the methanol and water from the reservoir to the anode, which made operation with very high concentrations of methanol, even neat methanol, possible. The maximum power density 24 mW cm−2 at room temperature obtained at 2 M without the porous plate was reproduced at 16 M with the porous plate. When high concentrations of methanol were used with the porous plate, it was confirmed that the Faraday efficiency remained high, and the back diffusion of water from the cathode to the anode through the membrane occurred which resulted in no flooding at the cathode, contrary to the case without the porous plate.
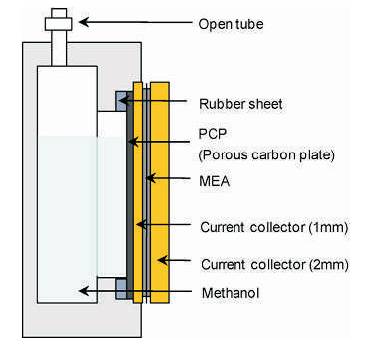
Figure 3. High concentrated methanol feed passive DMFC with porous carbon plate (PCP) as MBL (Abdelkareem & Nakagawa, 2006; Tsujiguchia & Abdelkareem, 2010)
Semi-passive DMFC, using porous polytetrafluroethylene [PTFE] plate was fabricated by Faghri et. al (2010), as shown in Figure 4. The effects of methanol barrier layer (MBL) thickness and electrolyte membrane thickness on cell performance, methanol and water crossover, and fuel efficiency had been studied. The results showed that a thicker MBL could significantly decrease the methanol and water crossover by increasing the mass transport resistance between the anode reservoir and the MEA, while a thinner Nafion® membrane could also significantly decrease the methanol and water crossover by enhancing the water back flow from the cathode through the electrolyte membrane to the anode. They were able to produce the maximum power density of 115.8 mW cm−2 when 20 M methanol solution was fed as fuel.
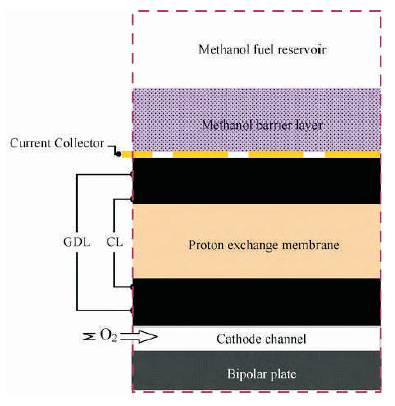
Figure 4. High concentrated methanol feed passive DMFC with polytetrafluroethylene [PTFE] as MBL (Faghri & Xu, 2010)
Wu et al. (2010) developed a microfluidic structured anode flow field for passive DMFCs (Figure 5) with neither liquid pumps nor gas compressors/blowers. The flow field was consists of plural micro flow passages. Taking advantage of the liquid methanol and gas CO2 two-phase counter flow, the unique fluidic structure enabled the formation of a liquid–gas meniscus in each flow passage. The evaporation from the small meniscus in each flow passage can lead to an extremely large interfacial mass-transfer resistance, creating a bottleneck of methanol delivery to the ACL. The fuel cell tests show that the innovative flow field allows passive DMFCs to achieve good cell performance with a methanol concentration as high as 18.0 M, increasing the specific energy of the DMFC system by about five times compared with conventional designs.
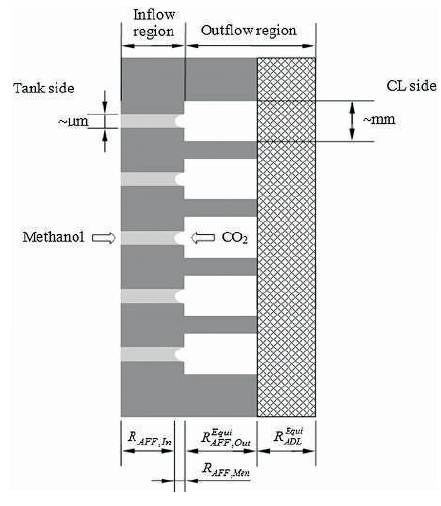
Figure 5. Micro-fluidic structured flow field used for achieving concentrated methanol operation of passive DMFC (Wu et al. 2010)
This article provides a review of past research efforts for achieving concentrated methanol operation of passive DMFC. For improving the energy density of the cell, it is important to operate the cell with concentrated methanol fuel. To achieve this, much of the research efforts had been directed towards developing low methanol crossover membranes and development of highly active anode catalysts. But these methods are having some serious disadvantage and thus not provide satisfactory performance improvement. Inserting a MBL between fuel reservoir and ACL emerges as a potential option and available results reveals its effectiveness in achieving the concentration methanol feed operation without affecting the cost. Although operating passive DMFC with concentrated methanol demands species (methanol, oxygen, water, CO2) management techniques different than those for conventional DMFCs. More research efforts are anticipated in this direction.