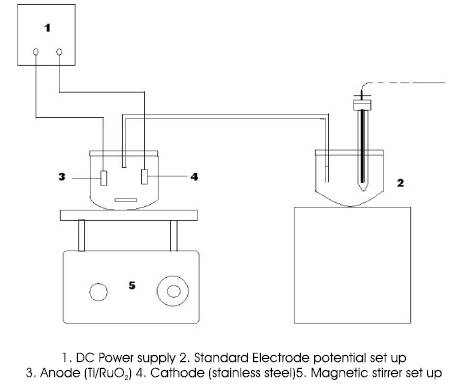
Figure 1. Constant stirring Batch Reactor Setup
The electrochemical destruction was carried with volumes (200,300,400 ml) of dissolved resin solution which act as electrolyte in electrochemical cell. In the electrochemical cell, the electrolyte used are
Anode : Ti/RuO2
Cathode : Stainless steel
Mediator : NaCl (g/l)
2.3 Experimental Procedure
The experiment was carried out in batch set up with different current densities of 1.25, 2.5, 3.75, 5 A/dm2 and it was run for 7 hrs. Sample of about 1ml were collected for every one hour and the temperature, pH, cell voltage, electrode potentials were measured.
The effluent volume of 200,300,400 ml was taken for the experiments. Electrolysis was carried out at different parameters such as current densities viz 1.25, 2.5, 3.75 and 5.00 A/dm2 with NaCl concentration as 8 g/l. During the electrolysis sample were collected at different time intervals.
2.4 Analysis
The collected samples were diluted and analyzed for Total Organic Carbon (TOC) present by using combustion TOC analyzer (FormacsHT). TOC analyzer was used to verify that no organics remained in the spent electrolytes. To determine the quality of organically bound carbon, the organic molecules must be broken down to single carbon units and converted to a single molecular form that can be measured quantitatively. Samples were withdrawn from the electrolysis solution at different intervals. They were filtered and acidified by HCl and brought to a pH two prior to analysis. They injection volumes were 50/100µL.The temperature in the oven was 680oC in combination with a Pt catalyst Calibration of the analyzer was achieved with potassium hydrogen phthalate standards (Merck)
Total Organic Carbon = Total carbon – Inorganic carbon.
3. Results and Discussion
Experiments were carried out at various volumes of electrolyte (200, 300, 400 ml). It was observed that good reduction in TOC (52.2 %) occurred at a current density of 3.75 A/dm2 for 200 ml of electrolyte, whereas at a current density of 5 A/dm2 the percentage reduction of TOC was found to decrease (Figure 2). For 300 ml of electrolyte the best reduction of TOC (73.8%) occurred at a current density of 3.75 A/dm2 (Figure 3), while for 400 ml of electrolyte the best reduction of TOC (63.7%) was found to occur at 3.75 A/dm2 (Figure 4) The current density was changed from 1.25 (A/dm2) to 5(A/dm2) and the best effect of TOC reduction was found to occur at 3.75(A/dm2) for the volumes of electrolyte. The concentration in the path decreased more rapidly leading to increasingly higher recovery with increase in the applied current density. Influence of current density: The electrolysis results taken with different current densities in the range 1.25 – 5 A/dm2 and the corresponding charge (Amps/Lt) for various volumes of electrolyte have been calculated and graphs thus plotted (Figure 5). The study revealed that the TOC reduction had only very little effect on change in volumes of electrolyte at the end of electrolysis, since the initial concentration of effluent was kept almost same.
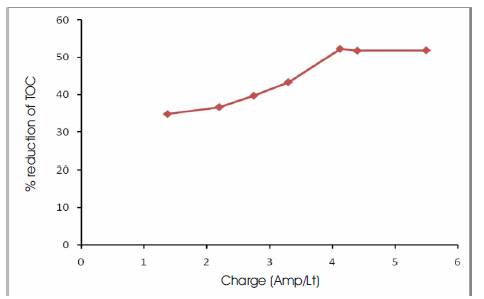
Figure 2. Percentage Reduction of TOC Vs Charge for 200 ml of Electrolyte
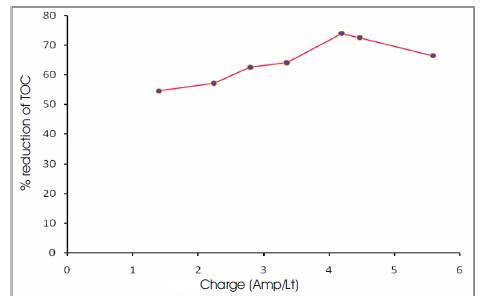
Figure 3. Percentage Reduction of TOC Vs Charge for 300 ml of Electrolyte
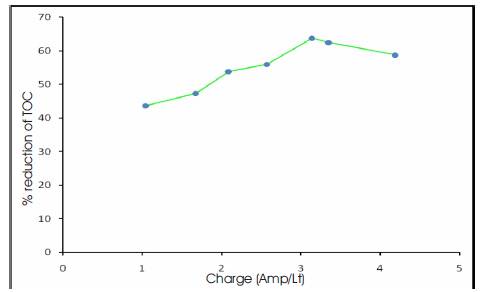
Figure 4. Percentage Reduction of TOC Vs Charge for 400 ml of Electrolyte
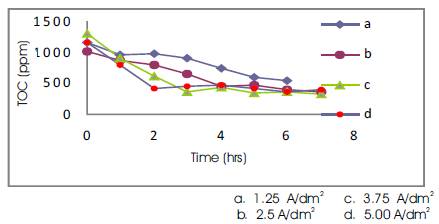
Figure 5. TOC Concentrations Vs Time for 300 ml Volume of Electrolyte.
Effect of electrode (anodic) potential: Figures 6 and 7 shows the effect of anodic potential for various current densities and volumes of electrolyte during the electrolysis time. Figure 6 depicts that the decrease in current density leads to decrease in the anode potential. Since, the anodic potential slightly increases during electrolysis due to the oxidation of organics on the anodic surface. Given a fixed current density and time duration, Figure 7 reveals that the anode potential decreases with increase in the volume of the electrolyte because of oxidation.
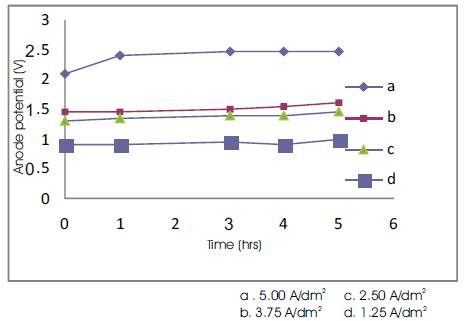
Figure 6. Anodic Potential Vs Time for 300 ml Volume of Electrolyte.
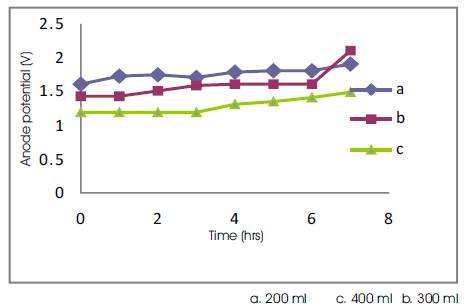
Figure 7. TOC Anodic Potential Vs Time for 3.75 A/dm2
Conclusion
This study focused in using electro chemical treatment based on MEO as a potential candidate to achieve mineralization of organic resins. The process is conducted for synthetic ion exchange resin materials by constant stirring batch reactor process. The experimental study was carried out in batch setup at different current densities 1.25, 2.5, 3.75, 5.00 A/dm2 for various flow rates 20, 40, 60, 80, 100 LPH using RuO2/Ti as anode and stainless steel as cathode in the electrolyser. The mediators used for this process are in situ generated OCl- ion and the Fenton's reagent (Fe2+/Fe3+ + H2O2),OHo. Thus NaCl acts not only as supporting electrolyte as well as enhances the anodic oxidation of organics. The study highlighted that in batch reactor set up the best effect of TOC reduction was found to occur at 3.75 A/dm2 with flow rate of 20 LPH.