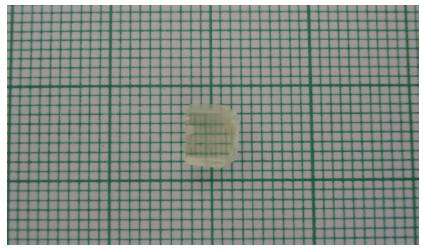
Figure 1. Urea-Organic Additive Single Crystals Photograph
Urea was grown from aqueous solutions with the organic additives by the slow evaporation method for obtaining an improved crystal size and enhanced nonlinear properties. The new material obtained was nonhygroscopic with remarkable morphological change compared to pure urea. The influence of the organic additives on the optical, structural, thermal and mechanical properties of urea were studied by UV in visible region, Fourier transform infra red spectroscopy, X-ray diffraction and microhardness studies by Vicker's hardness test. The second order nonlinear optical property was examined by Kurtz powder technique and is measured using Nd:YAG laser at 1064 nm with a beam energy of 3.2 mJ/pulse. This material grown as a single crystal has a widespread application in the field of optoelectronics engineering and technology.
Optical fiber communication system consists of optoelectronic devices at the stage of optical signal modulation. The quality of optical signal modulation primarily depends on the nature and the quality of optical materials used in the optoelectronic devices. Therefore it is of primary concern to employ high quality, high conversion efficient and pure single crystals in optoelectronic devices. The rapid growth in the field of optoelectronics has stimulated the searching for nonlinear organic crystals in order to perform efficient signal processing. The expansion of organic materials world for nonlinear optoelectronic devices is of enormous attention due to the higher orders of optical nonlinearities of those materials than the inorganic materials under practice. So it is vital to grow extremely perfect single crystals of organic nonlinear optical materials. Urea is one of the organic materials that posses good NLO properties due which it has been under practical application [1,2]. It is an interesting material and has aroused considerable interest amongst several researchers because of its high efficiency of wide frequency conversion and high damage threshold against high power laser [3]. Also, urea single crystal is of great interest for use in second harmonic generation and optical parametric oscillation. The exclusive properties such as transparency upto 200 nm wavelength and highly double refracting would be used for processes of frequency mixing and higher harmonic generation in a large range of spectrum including the ultraviolet spectrum [4-8]. Pure urea crystals grown from methanol are already reported to have better growth properties [9]. This work is carried out in view of growing perfect single crystals that can meet the requirement of high efficiency optoelectronic devices to perform the optical signal modulation with very low conversion losses in fiber optic communication system and optoelectronic switching circuits. A trial has been made to grow urea material by adding other organic materials such as m-nitrobenzoic acid, m-nitroaniline in a precise proportion to improve the optical transmission percentage of the crystal and to obtain better nonlinear properties [10]. The grown crystal was characterized and morphology, transparency and second order nonlinear analysed for interesting results with respect to the crystal properties. Urea due to the organic additives shows a considerable increase in the conversion efficiency and other nonlinear properties. It also shows a cutoff at 260 nm and hence best suited for NLO applications [11]. Therefore it may be a highly potential candidate in designing the future engineering and technology in the field of optoelectronics device fabrications.
[1,2] Reflection, refraction, diffraction and absorption are commonly observed interactions of light with matter. During these processes the optical properties of the material such as the refractive index and the absorption coefficient remain unaffected by the electromagnetic radiation. However when the light used is in the form of powerful laser beams, some materials manifest marked changes in their optical properties as a result of the interaction with the strong electromagnetic field of the radiation. This in turn effects a modification of the frequency, phase or amplitude of the light transmitted through the material. Such interactions arising out of multiphoton effects are known as nonlinear optical (NLO) processes and the materials in which such processes can be carried out efficiently are called NLO materials.
[3] High power laser light photons when interacts with nonlinear materials such as urea crystals, they effectively combine to form new photons with twice the energy, therefore twice the frequency and half the wavelength of initial photons; known as frequency doubling or in general frequency conversion.
[4-8] The urea crystal is transparent to ultraviolet radiations upto 200 nm wavelength. They are highly double refracting in nature. Therefore, urea crystal finds a wide application in the process of frequency mixing. In this process, the sum or difference of two or more (three) input frequencies is obtained in the output when passed through NLO materials. These crystals are also capable of producing higher harmonic generations such as three times or four times the input frequencies. The nonlinear efficiency of molecular organic crystals is seen to depend on the molecular hyperpolarizability and on the crystalline geometry. The light modulation takes place in this fashion when they traverse through the nonlinear materials.
[9] Single crystals of pure urea have been grown from methanol as solvent by solvent evaporation method.
[10] A change in the optical property of a material in response to an electric field that varies slowly compared with the frequency of light is the electro-optic modulation process. The simplest kind of electro-optic modulation consists of a crystal whose refractive index is a function of the strength of the local electric field. If the nonlinear crystal such as urea is exposed to an electric field, the light will travel more slowly through it. But the phase of the light leaving the crystal is directly proportional to the length of time it took that light to pass through it. Therefore the phase of the laser light exiting the electro-optic modulator can be controlled by changing the electric field in the crystal.
[11] Various nonlinear optical application due to the nonlinear processes such as electro-optic modulation, frequency conversion, optical phase conjugation and optical solitons analysis are achieved using urea single crystals.
The synthesis of the compound was carried out by mixing urea, m-nitrobenzoic acid and m-nitroaniline in 10:1:1 in ethanol. All the reactants taken were pure analytical reagents(Merck, 99.5% pure). The homogeneous solutions thus formed was filtered out and kept undisturbed at room temperature in a closed beaker with provisions for controlled evaporation. After a period of 10 days, crystalline material of Urea-Organic additive compound was separated out.
Urea-organic additive compound were grown into a single crystals by slow evaporation technique at room temperature. Ethanol was chosen to be the suitable solvent for preparing the growth solutions. Urea-Organic additive compound was dissolved in ethanol until it has reached the saturation point. The solution was filtered and kept in a closed beaker at room temperature. The rate of evaporation of the solvent was controlled critically for a period of 45 days. Single crystals of urea-organic additive were harvested on the 50th day (Figure 1). The grown crystals were found to exhibit birefringence (Figure 1) property when visually examined.

Figure 1. Urea-Organic Additive Single Crystals Photograph
Morphological studies were conducted on the urea crystal with organic additives. It has a well defined and developed morphology with many predominant habit faces. The morphology shows that all the faces are not equally developed in the crystal. The morphology of the urea crystal grown with organic additives is entirely different from the morphology of pure crystals[9]. The crystal showing double refraction is clearly visible from the Figure 1. Every single line on the graph sheet appears to be splitted up into two lines due to double refraction. One being the ordinary ray and other the extraordinary ray.
To obtain the unit cell parameters and to confirm the crystallinity of grown crystals, both single crystal and powder crystal X-ray diffraction were carried out. Powder X-ray diffraction pattern was recorded using a microprocessor controlled X-ray diffractometer with 1.540598 Cu wavelength as the target in reflection scan mode using scintillation counter detector. The powder XRD analysis shows the Bragg's reflection at the lattice planes of urea-organic additive crystal is shown in the Figure 2.
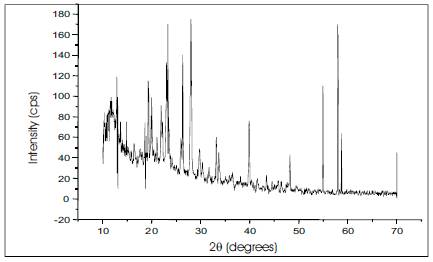
Figure 2. Powder XRD of Urea-Organic Additive Single Crystals
The UV-visible spectroscopic study of the grown urea-organic additive single crystal of 1.5 mm size was done using PerkinElmer Lambda 35 UV Spectrophotometer. The optical transmission range was determined for the crystals. To know the suitability of urea-organic additive single crystals for their use in electro-optic modulation in the region extending from 200 to 1100 nm covering the ultraviolet region, the transmission spectra was taken at room temperature. The Figure 3 shows the optical transmission spectrum of urea-organic additive single crystals grown from ethanol. The crystal is optically transparent in the UV-Visible region with good transmittance which implies that the urea in the presence of other organic additives is found to be a best candidate for electro-optic modulation process. It shows a cutoff at 260 nm and hence best suited for NLO applications.
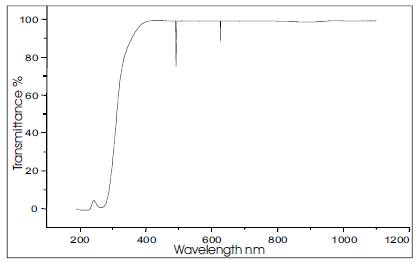
Figure 3. UV Visible Transmission Spectrum
Urea-organic additive crystal was analysed by FTIR spectroscopy using PerkinElmer Spectrum FT-IR spectrometer at room temperature to identify the incorporation of functional groups of the organic additives in urea (Figure 4). The incorporation of m-nitrobenzoic acid, m-nitroaniline into urea is confirmed by this analysis.
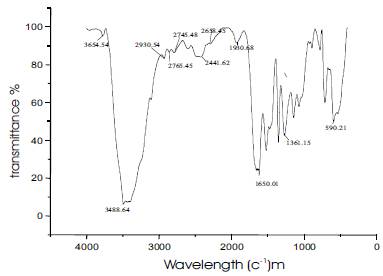
Figure 4. FT-IR Spectrum of Urea-Organic Additive Single Crystal
Alcohols and amines display strong broad O-H and N-H stretching bands in the region 3400-3100 cm-1. The bands are broadened due to hydrogen bonding and a sharp 'non-bonded' peak are seen at around 3486 cm-1. Alkene and alkyne C-H bonds display sharp stretching absorptions in the region 3100-3000 cm-1. The bands are of medium intensity and are often obscured by other absorbances in the region (i.e., OH).
Triple bond stretching absorptions occur in the region 2400-2200 cm-1. Absorptions from nitriles are generally of medium intensity and are weakly defined. Alkynes absorb weakly in this region as they are highly asymmetric.
Carbonyl stretching bands occur in the region 1800-1700 cm-1. The bands are generally very strong and broad. Carbonyl compounds which are more reactive in nucleophilic addition reactions (acyl halides, esters) are generally at higher wave number than simple ketones and aldehydes, and amides are the lowest, absorbing in the region 1700-1650 cm-1.
C=C double bond stretching occurs in the region around 1650-1600 cm-1. The bands are sharp and of medium intensity. Aromatic compounds typically display a series of sharp bands in this region. Carbon-oxygen single bonds display stretching bands in the region 1200-1100 cm-1. The bands are strong and broad. All the above confirms the presence of functional groups of all the organic materials present in urea-organic additive single crystal.
The Kurtz's and Perry powder test was performed to study the NLO property of urea-organic additive single crystal. The sample was taken as powder and was irradiated by pulsed beam of Nd:YAG laser of 8ns pulse width with a repetition rate of 10 Hz and scattering geometry of 90 degree. The beam energy of 3.2 mJ/pulse was used and the slit width was kept at 1mm. The second harmonic beam was focused by a lens of focal length 20 cm and measured using Tektronix TDS 3052B Oscilloscope. Hamamutsu R2059 Photo Multiplier tube with a power supply of 2.5 kV/mA was used. The emission of green radiation by the sample confirms the second harmonic generation of the grown material. It has an SHG efficiency of 1.25 times that of pure urea due to organic additives.
Vicker's Microhardness number was used to evaluate the anisotropy in mechanical hardness for urea-organic additive single crystal of thickness 3mm employing a SHIMADZU (HMV2T) microhardness tester for different loads. The selected smooth surfaces of the crystal were subjected to Vicker's static indentation test at room temperature by applying loads ranging from 25 – 200g. The hardness (HV ) was calculated using the relation
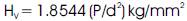
Where P is the applied load and d is the diagonal length of the indentation impression.
For a particular load, at least five well-defined impressions were considered and the average of all the diagonals was considered. The Vickers hardness number (HV) was calculated using the relation HV = 1.8544 (P/d2) kg/mm2. Figure 5 shows the variation of HV as a function of applied load. It is clear from the Figure that HV decreases with increase of load.
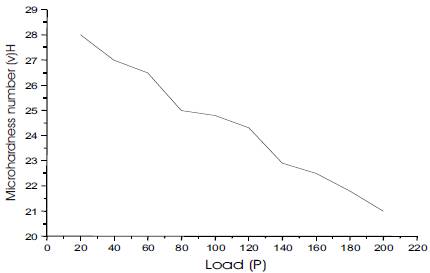
Figure 5. Variation of Microhardness Number (HV) with load (P) of Urea-Organic Additive Crystals
The urea-organic additives material was synthesized and crystallized by slow evaporation solution growth method at room temperature. Due to the organic additives, the growth parameters of urea have shown an enhancement in its properties. The grown crystal has a well defined morphology and therefore it can be grown into bulk crystals. Unlike pure urea crystals, the grown urea-organic additive crystal was found to be non-hygroscopic. The crystal also shows double refraction. The FT-IR spectrum shows the incorporation of different functional groups of organic additives. The variation of Vickers hardness number (HV) as a function of applied load shows a decrease with increase of load. The SHG efficiency of grown crystal is 1.25 times that of pure urea due to which the crystal may be used for laser devices. The transparency in the entire visible region facilitates this material to various optical modulation processes. With all these added advantages of this urea-organic additive material, it is evident that this material may a potential candidate in the fabrication of optical devices for the optical modulation process and this approach of organic additives may be a breakthrough in the future engineering and technology in the field of optoelectronics and switching device fabrication.
Hereby the author state that the above work is the original work done by the author as a contribution to research and technology. The authors thank various research centres like, IISc, Bangalore, Madurai Kamaraj University, Madurai for their support for characterization of the crystal samples. The authors also thank the parent Institutions in the title for their encouragement and guidance throughout the research work.