
Bubble column reactors are intensively used as multiphase flow reactors and reactors in chemical ,biochemical and petrochemical industries. Till recently its visionary potential is exploited in an effective and far-reaching manner in treating textile dye effluents, thus opening the window of innovation in the field of application of environmental engineering science. These reactors provide several advantages and benefits during operation and maintenance such as high heat and mass transfer rates, compactness and low operating and maintenance costs. Three phase bubble column reactors are widely employed in reaction engineering,i.e. in the presence of catalysts and in the wide avenue of biochemical engineering. Textile waste effluents are one of the wastewaters that are difficult to degrade by primary and secondary treatment procedures and they contain recalcitrant compounds. So the need of a tool as a tertiary treatment process-ozone-oxidation or advanced oxidation process for treating textile dyeseffluents in a bubble column reactor. The aim of this study is to gain understanding and unravel the hidden secrets of the tool of bubble column reactor in degrading textile dye effluents. Fundamentals of the subject striving towards applied aspects of the ozone technology and its application are presented in this study with minute details.
In the second stage an attempt has been targeted to explore the kinetics of the ozone oxidation of dyes and the subsequent design of bubble column reactor. A particular pH and a particular redox potential will be found for a specific dye conversion of dye. A number of different types of dyes will be utilised-anthraquinone and azo dyes. It will open up a new area of environmental engineering science.
Application of nanotechnology in environmental engineering science is also a vision and mission of the study and research. Nanotechnology can be extensively used in developing safe drinking water for a growing population. The application of nanotechnology to the purification and treatment of water supplies to make them potable may potentially revolutionize water purification and treatment. The future vision leads towards this area of innovation.
Bubble column reactors extensively belong to the general class of multiphase reactors which comprise of three main avenues, namely, the trickle bed reactor(fixed or packed bed),fluidized bed reactor and the bubble column reactor. The definition of a bubble column reactor-a cylindrical vessel with a gas distributor at the bottom. The gas is distributed and sparged in the form of bubbles into either a liquid phase or a liquid-solid suspension. These type of reactors are called slurry phase reactors when a solid phase exists. Bubble column reactors are widely and extensively used as multiphase contactors and reactors in chemical, petrochemical, biochemical and metallurgical industries. The vision of its utilization is such that it is widely used in reactions such as oxidation, chlorination, alkylation, polymerization and hydrogenation, in the manufacture of synthetic fuels by gas conversion processes and in biochemical processes such as fermentation and biological wastewater treatment(Lin,2003).
Dye wastewater cannot be degraded by primary and secondary treatment procedures (Konsowa,2003) (Lackey,2006). Ozonation or ozoneoxidation is the only other alternative. This study deals with ozonation of Direct Red dye (Direct Red 23 dye) in a bubble column reactor with the influence of different process parameters such as pH and redox potential. It has been proved that the rate constant and order of reaction depends tremendously on pH of the dye solution and also the oxidation-reduction potential(Table1).
There have been several improvements and innovations in the wastewater treatment field in the last few years. Alternatives have proved themselves for classical and conventional wastewater treatment systems. Advanced wastewater treatment systems have become a visionary global focus as individuals, scientific and non-scientific communities, industries and nations strive and surge forward to keep essential resources available for use. Advanced wastewater treatment techniques, coupled with wastewater reduction and water recycling initiatives offer avenues to the proper utilization of usable water. A scientist's and a common man's vision is widened by the invaluable expertise of scientific community.
A wide range of compounds is detected in industrial and municipal wastewater. Some of these compounds (both synthetic organic compounds and naturally occurring substances)pose severe problems in biological treatment systems due to their resistance to biodegradation or/and toxic effects on microbial processes. As a result and an effective conclusion, the use of alternative treatment technologies, aiming to mineralize or transform refractory molecules into others which could be further biodegraded, is a matter of great concern. Among them, advanced oxidation processes(AOPs) have already been used for the treatment of wastewater containing recalcitrant organic compounds such as pesticides, surfactants, colouring matters, pharmaceuticals and endocrine disrupting chemicals. Moreover, they have been successfully used as pre-treatment methods in order to reduce the concentrations of toxic organic compounds that inhibit biological wastewater treatment methods.
The main mechanism of AOP's function is the generation of reactive free radicals (Chen,2003). Hydroxyl radicals(HO') are effective in destroying organic chemicals because they are reactive electrophiles (electron preferring) that react rapidly and non-selectively with nearly all electron-rich organic compounds. They have an oxidation potential of 2.33 volts and exhibit faster rates of oxidation reactions compared to conventional oxidants such as H2O2 or KMnO4 (Figure2).
Bubble column reactors belong to the general class and division of multiphase reactors which consist of three main categories namely, the trickle bed reactor (fixed or packed bed), fluidized bed reactor, and the bubble column reactor. The application of advanced oxidation process in bubble column reactor has tremendous potential (Lin,2003). Bubble column reactors owe their wide application area to a number of advantages they provide both in design and operation as compared to other reactors. First of all, they have excellent heat and mass transfer characteristics, meaning high heat and mass transfer coefficients (Kantarci,2005). Little maintenance and low operating costs are required due to lack of moving parts and compactness.
Wastewater may be classified into four categories:
The oxidation- reduction potential of ozone is 2.07 volts and of hydroxyl radicals is 2.8 volts (Chen,2002). So this high redox potential helps to degrade many recalcitrant chemicals as well as dyes. Ozonation takes place with the help of nascent oxygen atom in the oxygen molecule. According to literature (Chu,2000), at lower pH the ozonation takes place with the help of molecular ozone and at higher pH the ozonation takes place with the help of hydroxyl radicals. Besides reaction kinetics in the past and in literature showed that order of reaction is pseudo zero order before ozone concentration is reached and tends towards first order when ozone concentration reaches a saturation level. (Ravikumar,2005).
The study tackles the kinetics of the ozonation reaction and the final conclusion being that the order varies extensively with the change of pH of solution. They also showed the conversion levels at different pH of dye solution.
Since the oxidising ability of ozone comes either from molecular ozone or hydroxyl free radicals, the rate of dye disappearance can be formulated as follows (Chu et al,2000):

Where [D] is the concentration of dye in the solution, [O3 ] and [OH’] are the concentrations of ozone and hydroxyl radicals, ko and kOH are the respective kinetic rate constants. In a particular study where there is saturated ozone concentration or in excess, the hydroxyl free radicals and ozone concentration in the solution are presumably close to constants(i.e. at steady state).Therefore equation (1) can be rearranged to the pseudo first –order equation in equation(2) where k is the overall pseudo first-order rate constant (Chu et al,2000).
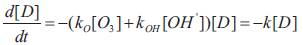
Our investigation lies in finding whether the order of Reaction is first order in saturated ozone concentration (Prasad,2009). So the rate equation of examination is
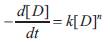
k = rate constant, n = order of reaction
The assumption in addressing this equation is that ozone concentration is saturated
In this research, the author have done ozonation of dye(Direct Red 23 dye) in a 500ml Perspex cylindrical flask. Our future target is to design a bubble column reactor for this particular type of ozonation reaction (Ruzicka,2001). The ozone output in the ENALY –CHINESE made ozonator is 200-300mg/hr. Our vision and scheme of research is as follows:
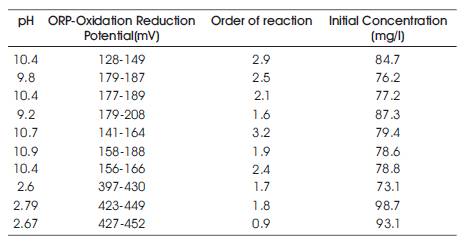
Table 1. Data showing the variation of order of reaction with pH and redox potential
The dye taken was Direct Red 23 dye, a diazo dye which is soluble in water. Later in our ongoing research, dye ozonation kinetics of five different dyes will be attempted and pursued (Srinivasan, 2009).
In the beginning of our research, dye ozonation kinetics of initial dye concentrations of 90mg/l,100mg/l,120mg/l and 135mg/l was pursued. Then a particular initial concentration of 100mg/l was taken and the kinetics at pH 3,7 and 9 are minutely investigated. Samples were collected from the cylindrical flask at times of 5,10,15,20,25,30,35,40,45 and 50 minutes, then the samples were tested for their absorbances with the help of UV spectrophotometer(UNICAM made).
Before measurement of concentration, a calibration chart was made between absorbance and concentration.
The relation between absorbance versus concentration is as follows (Figure 1).
Y=k1 x+k2 ,where k1 =0.0332 ,k2 =-0.3654
The rate of dye degradation with time is evaluated with the help of Newton's Forward Interpolation Formula. Then we follow the following rate equation:
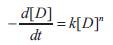
Taking logarithm on both sides of the equation we get
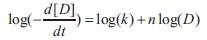
The rate constant (k) and order of reaction (n) are analysed by EXCEL LINEST function(least squares regression analysis function).

Figure 1. Absorbance vs Concentration(Calibration Curve)
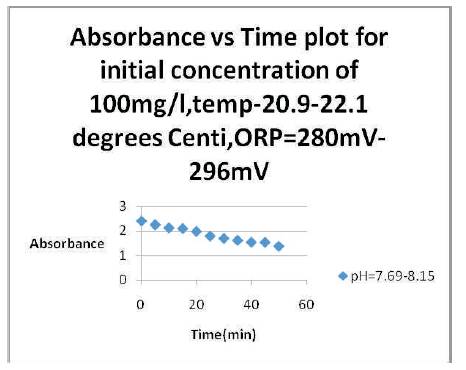
Figure 2. Variation of absorbance vs time at different temperature and Oxidation-Reduction Potential
Nanofiltration is a far-reaching avenue of reaching. Scarcity of water is a problem in every developing countries of the world. Increasing cost of water and its profligate consumption necessitate a treatment process that is integrated with in-plant water circuits rather than as a subsequent treatment. From this view, membrane filtration offers potential applications. Processes using membranes provide very interesting possibilities for the separation of hydrolyzed dye-stuffs and dyeing auxillaries that reduce colouration and BOD/COD of the wastewater. The choice of the membrane process ,whether it is reverse osmosis, nanofiltration, ultrafiltration or microfiltration must be guided by the quality of the final product.
Ozonation or ozone-oxidation is a very important and premier innovation of thought. A scientist and environmental engineer's vision is widened while pursuing this area of scientific pursuit. Advanced oxidation process is the only alternative for the tertiary treatment of recalcitrant dye molecules(Beltran,1997). Research in this area is not new yet immature. The vision in this area is innovative and brilliant. Lot of research in the design of bubble column reactor will open up a new era in environmental engineering.
The author would like to acknowledge School of Planning, Architecture and Civil Engineering, Queen's University, Belfast, United Kingdom, Dr. Bhaskar Sengupta, Dr. Des Robinson and Dr. John Mckinley to give me a chance to do the experiments in their laboratories and I am extremely indebted to their help.