Figure 1. Difference Between EEG vs. MRI vs. fMRI
Computer vision, autonomous driving, natural language processing, and speech recognition are just a few of the industrial and research disciplines that are being transformed by Artificial Intelligence (AI) and Machine Learning (ML) approaches. The availability of automated solutions may improve the precision and reproducibility of the execution of crucial activities in a variety of sectors, including radiology, diagnostics, and many others, where it is already having a significant impact. Paralysis occurs when people are unable to make voluntary muscle movements. Paralysis is caused by a problem with the nervous system. This paper focuses on the study of the contribution of Artificial Intelligence in identifying paralysis and explores the idea of building a Paralysis Prediction and Monitoring Model (PPAMM) with the help of AI and ML techniques. It is used to determine the frequency of nerve stimulation in the affected region as well as monitor the stimulus and paralysis-precipitating factors.
Paralysis is a loss of motor function in one or more muscles. Paralysis can be accompanied by a loss of feeling in the affected area if there is sensory damage as well as muscle damage. The motor route refers to the network of nerve cells that extends from the brain to the spinal cord and out to the muscle. Intact connections along this motor route are necessary for normal muscle function. The brain's capacity to regulate the muscles' motions is diminished by injury at any moment. Paresis, another word for weakness, results from this decreased efficiency. Any willed movement is completely impossible in the absence of communication. It refers to this loss of control as "paralysis." Periodic paralysis, in which weakness appears and disappears, is brought on by certain hereditary defects in muscle. A subset of Aritificial Intelligence (AI) called Machine Learning (ML) enables learning from prior experience or data without preprogramming. AI is a technology that mimics human intellect. The basic objective of AI is to develop a clever computer system that can solve diverse problems by functioning like a human brain (Habeeb, 2017). There are traditional methods to treat paralysis, like rehabilitation programs that include physical therapy, occupational therapy, and surgical methods. Recently, Artificial Intelligence was used to surgically treat paralyzed patients.
The goal of AI is to create intelligent machines. It has evolved into an important component of the technology industry. Artificial Intelligence research is very technical and specialized. Programming computers for specific traits is one of the central issues in Artificial Intelligence. Knowledge, reasoning, problem solving, perception, learning, planning, and so on are examples of cognitive abilities. Object manipulation and movement ability AI is built around knowledge engineering research (Zhang et al., 2014). Machines can frequently act and react like humans if given a large amount of information. Concerning the world, Artificial Intelligence requires access to objects, categories, and knowledge. To implement knowledge engineering, its properties and relationships with other knowledge must be established. It is difficult to instill common sense, reasoning, and problem-solving ability in machines. The use of Machine Learning models to search medical data and uncover insights to help improve health outcomes and patient experiences is known as Artificial Intelligence in medicine. Artificial intelligence is quickly becoming an integral part of modern healthcare, in both computer science and informatics. AI algorithms and other AI-powered applications are being used to assist medical professionals in clinical settings and ongoing research (Chan et al., 2018).
Medical Artificial Intelligence (MAI) is primarily used to per form clinical diagnoses and treatments. In recommended treatments, AI has the ability to detect meaningful relationships in a dataset and is widely used in a variety of clinical settings to diagnose, treat, and forecast outcomes. In medical AI research and studies, people are particularly interested in the viability and feasibility of incorporating various computer AI techniques into medical information modeling and clinical procedure deployments. AI techniques are being applied to pragmatic clinical procedures and analytical medical informatics, particularly in the following areas,
As a result of AI methodologies' significant capabilities and capacities in the recognition of meaningful data patterns, tools for clinical trials have been widely experimented with, particularly to aid decision-making in each phase for diagnoses and subsequent treatments, as well as prognoses and projections.
Integrating medical AI into clinician workflows can provide important context for providers to make care decisions. A trained Machine Learning algorithm can help clinicians save time on research by providing them with valuable search results that include evidence-based insights about treatments and procedures while the patient is still present.
There are numerous potential ways for AI to reduce healthcare costs. Some of the most promising opportunities include reducing medication errors, providing customized virtual health assistance, preventing fraud, and facilitating more efficient administrative and clinical workflows.
Many patients have questions after normal business hours. Chatbots that can answer basic questions and provide patients with resources when the provider's office is closed can help provide around-the-clock support. AI could also be used to triage questions and flag information for further review, alerting providers to health changes that require additional attention.
One significant advantage of deep learning is that AI algorithms can use context to differentiate between different types of data. A well-trained AI algorithm, for example, can use Natural Language Processing (NLP) to identify which medications belong in the patient's medical history if a clinical note includes a list of a patient's current medications as well as a new medication that their provider recommends.
Journal of Nature Medicine, published a research conducted by Grégoire Courtine and Jocelyne Bloch of the Swiss Federal Institute of Technology in Lausanne. It contributes to the formation of the technology business “Onward Medical” in the Netherlands, which is attempting to market the system. Researchers announced that three patients with spinal cord injuries who had entirely paralyzed lower bodies were able to walk, cycle, and swim with the use of a nerve-stimulation device managed by a touchscreen tablet. One to nine years prior to obtaining therapy, the patients' injuries to the thoracic spine, which is located below the neck and above the lowest part of the back, had occurred. After neurosurgeons first inserted prototypes of a nerve stimulation device remotely controlled by Artificial Intelligence software, it was able to take its first steps within an hour. By operating the nerve-stimulation devices using a remote control over the course of the following six months, the patients regained the capacity to partake in more difficult activities, such as walking, cycling, and swimming, in public places outside of the clinic (Rabinovitch, 2022).
Brown University, one of the world's premier educational and research institutes, has joined hands with Intel to undertake a research project that uses Artificial Intelligence to help solve paralysis in patients induced by spinal cord injuries. The project, backed by $6.3 million in funding from the United States' Defense Advanced Research Projects Agency (DARPA), aims to develop what is being called an 'Intelligent Spinal Interface' – a technology that may help "restore limb movement, sensation, and bladder control for people who have suffered severe spinal cord injuries" (Das, 2019).
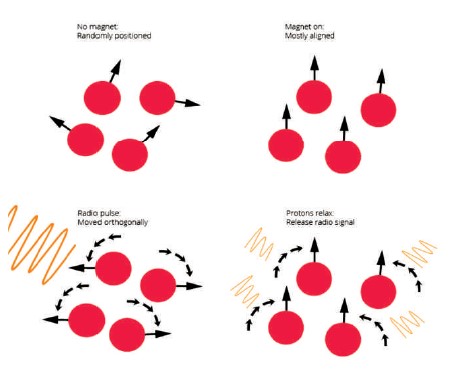
Magnetic Resonance Imaging (MRI) provides a map of the brain—how it looks at a set moment in time. This structural information can be useful for determining how the sizes of certain brain areas compare across people or if there is something abnormal about a particular brain area. Magnetic Resonance Imaging (MRI) relies heavily on magnets, as the name suggests, but these magnets are much more powerful than typical refrigerator magnets, being between 1,000 to 3,000 times stronger. The human body is made up of 70% water, so there are plenty of hydrogen atoms for the magnet to influence. The magnetic field from the MRI interacts with the protons in hydrogen atoms. These protons are usually randomly facing, but the magnetic field forces a significant portion of them to orient in the same direction. So, when people lie in an MRI machine, the protons in the hydrogen atoms in the body water basically point in the same direction (IMotions, 2019).
The protons are then effectively turned to one side by the radio pulse that is released in the following phase. However, because the radio frequency only lasts for a brief period of time, the protons relax and return to their initial alignment. The most important part is that once the protons relax, energy is released that the MRI machine's sensors can pick up on, as shown in Figure 1. The computer can figure out how the tissue appeared based on this energy that is released through some calculations.

Figure 1. Difference Between EEG vs. MRI vs. fMRI
Electroencephalography (EEG) uses electrodes attached to the scalp to track the electrical activity of the brain, as shown in Figure 2. From the surface measurements, it reveals the level of brain activity. This can be helpful for swiftly detecting how the brain responds to stimuli and for measuring aberrant activity, such as with epilepsies. The brain is an electrical system that captures all ideas, whether conscious or not, through a network of neurons that communicate with one another via electrical currents. More electrical signals result in increased neural transmission, which in turn increases brain activity (Farnsworth, 2022).
Instead of detecting changes in a single neuron, an EEG headset's electrodes look for electrical changes in thousands of neurons that are signaling at once. Following that, an amplifier receives the signal from the electrodes and amplifies it, as expected. When a computer receives this input, it can quickly and accurately produce a variety of maps of brain activity.
Figure 2. EEG Biofeedback in Dallas: Neurofeedback for Better Brain Balance
Functional Magnetic Resonance Imaging (fMRI) is a class of imaging techniques designed to demonstrate regional, time-varying changes in the brain. To deliver the command to carry out this action, a specific section of the brain will become more active and receive a tiny bit of more oxygen-rich blood. The calculations for fMRI are similar to those for MRI in that the energy emitted by the relaxation of protons is measured, but they are focused on figuring out how the amount of oxygenated blood flow fluctuates, as shown in Figure 3. One can assume that a certain area of the brain is more active if it has more oxygenated blood than other areas. This is referred to as the "Blood Oxygen Level Dependent" (BOLD) reaction. The temporal resolution of fMRI has a disadvantage. The data gathering is delayed because it takes several seconds for the blood flow to alter and computational constraints limit the actual recording.
Figure 3. fMRI
This technique aims to stabilize nerve impulses after a patient becomes paralyzed. Ali et al. (2015); Dhamanda et al. (2021) the idea is to develop software based on Artificial Intelligence and Machine Learning techniques that monitors the symptoms, forms a database to predict the occurrence of paralysis, and examines the minute nerve signals transmitted on affected nerves. This collected data will be very useful to doctors in suggesting alternative medications.
Step 1: Start
Step 2: Automatically collect the Paralysis Precipitating Factors Data (PPFD) or manually enter the PPFD into the device. PPFD includes symptoms like blood pressure and heart rate.
Step 3: Monitor the nerve stimulus and collect the data, like Nerve Stimulus Data (NSD)
Step 4: Store the collected PPFD and Nerve Stimulus Data in the database.
Step 5: Merge both PPFD and NSD
Step 6: Using AI and ML, process the cumulative PPFD and NSD and predict the possibility and occurrence of paralysis.
Step 7: Based on the result, decide whether to show an alert or not.
Step 8: Display the alert message and stimulus frequency on the device monitor.
Step 9: Stop.
After installation of an AI-based software model into Internet of Things (IoT) devices such as smart watches and bands, it automatically collects Paralysis Precipitating Factors Data (PPFD) such as blood pressure (Sidey- Gibbons & Sidey-Gibbons, 2019; Olunu et al., 2018), hyperlipidemia, and sugar levels. In some cases, it can enter the data manually. The microbiosensor, which can identify nerve impulses (electrical signals), is always on and monitors nerve impulses in the paralyzed area, collecting Nerve Stimulus Data (NSD). The database stores the merged PPFD and NSD. Kumar et al. (2019b) Using AI and ML, it predicts the possibility of paralysis using the cumulative PPFD and NSD (IBM, 2020a; IBM, 2020b). If the probability is high, an alert message will be displayed on the screen along with the frequency of the nerve stimulus (Kumar et al., 2019a). The medication can be adjusted based on the frequency, PPFD, and NSD. The entire workflow of the Paralysis Predicting and Monitoring Model (PPAMM) is illustrated in Figure 4.
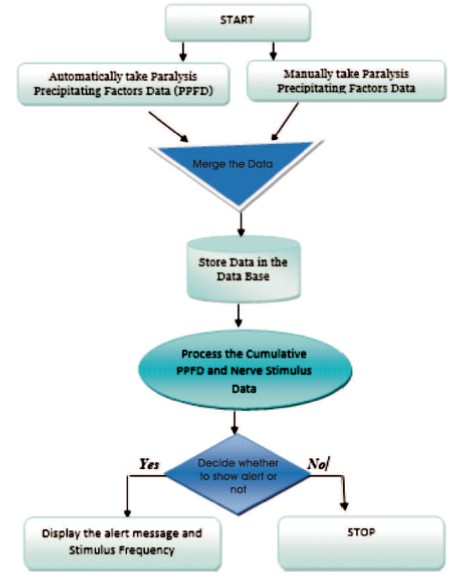
Figure 4. Paralysis Prediction and Monitoring Model
The proposed model is based on a hypothesis, and the practical implementation of the model raises many questions, such as about algorithm usage and the scope of input data. The challenging part is selecting a Machine Learning algorithm from its wide range. The size, nature, and quality of the data, as well as the computational time that is available, the urgency of the task, the size of the data set, and the purpose for which the data will be used, are more influential factors in implementation.
Bayesian algorithms might fit better in the proposed model as a set of algorithms that all follow the same principle, namely that each pair of features being classified is independent of the others. The term "Naive Bayes classifiers" refers to a set of classification algorithms based on Bayes' Theorem. The relationship between P(A|B) and P(B|A) is described by the Bayes formula.
Additional features like,
Paralysis Predicting and Monitoring Model (PPAMM) will make complex things simple. A normal human can understand what is happening in the paralyzed body part. The alert feature will be an effective mode of communication among the patient, doctor, and caretakers.
An MRI is costly due to the enclosed nature of the machine which can cause feelings of claustrophobia in some patients. There is a chance of developing allergies to the gadolinium contrast medium, commonly known as the contrasting reagent. The main drawback of Electroencephalography (EEG) recording is the lack of spatial resolution. It will get a comprehensive output in the proposed invention as it is based on Artificial Intelligence, and this technology will be installed in wearable devices like smart watches and smart bands, so patients will not need to go into heavy machines for the scans. It can use this device to monitor the nerve stimulus of the paralyzed area and then adjust the medication based on the results.
This device will help the doctors to decide how to treat patients. The current method necessitates further research into nerve signal transmission and the development of a micro biosensor capable of detecting electric signals in the human body. This method can use techniques such as deep neural networks, deep learning, data mining, and data science to modify the algorithm.