Figure 1. Lung Anatomy
Segmentation of the pulmonary lobes is relevant in clinical practice and particularly challenging for cases with severe diseases or incomplete fissures. In this work, an automated segmentation approach is presented that performs a transformation on Computed Tomography (CT) scans to subdivide the lungs into lobes. Content- based image retrieval has been a major research area with major focus on features extraction, due to its impact on image retrieval performance. When applying this in the medical field, required different feature extraction method that integrate some domain specific knowledge for effective image retrieval. Here a novel method called atlas based segmentation is proposed. Atlas methods usually require the use of image registration in order to align the atlas image or images to a new, unseen image. This method provides complementary information from past cases with confirmed diagnoses, to lung tissue classification and quantification in CT images. The system exploits the location of the pathological lung tissue and allows significant improvement in terms of early retrieval precision when compared to the approach based on global features only.
Segmentation of pulmonary lobes from Computed Tomography (CT) images is a precursor to most pulmonary image analysis applications. Computed Tomography (CT) for the body has been available since 1975. Medical Image Processing is currently a hot research area in medicine and it is believed that they will receive extensive application to biomedical systems in the next few years.
Segmentation of the pulmonary lobes is relevant in clinical practice and particularly challenging for cases with severe diseases or incomplete fissures. In this work, an automated segmentation approach is presented that performs an atlas-based localisation on Computed Tomography (CT) scans to subdivide the lungs into lobes.
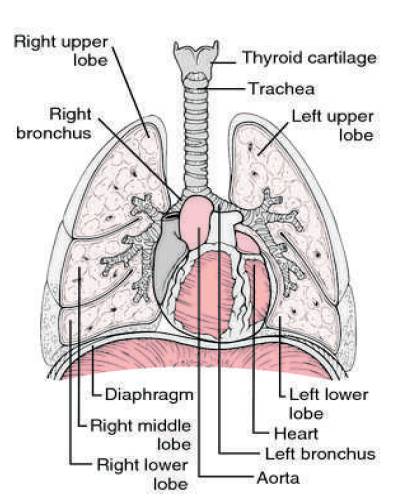
As in Figure 1, the human lungs are subdivided into five lobes that are separated by visceral pleura called pulmonary fissure. There are three lobes in the right lung, namely upper, middle, and lower lobe.
The right upper and right middle lobe are divided by the right minor fissure where as the right major fissure delimits the lower lobe from the rest of the lung. In the left lung there are only two lobes, the upper and the lower lobe, that are divided by the left major fissure. The characteristic of the pulmonary lobes are separated supply branches for both vessels and airways. Lung lobe segmentation is relevant in clinical applications particularly for treatment planning. The location and distribution of pulmonary diseases are important parameters for the selection of a suitable treatment. Computed Tomography (CT) allows visualization of the lungs within a few seconds. Since typical scans with high anatomical details contain over 400 slices with sub millimetre resolution for each direction, manual segmentation is time consuming and there is demand for automatic lung lobe segmentation methods. The segmentation of pulmonary lobes is challenging because of anatomical variation and incomplete fissures. On the one hand, pathologies can deform the lobes and make the fissures unrecognizable. Water shed methods are generally time-consuming; segmentation of one case took 2 hour on average.

Figure 1. Lung Anatomy
Another disadvantage of this approach was that scans with lobar shapes not represented in the data set were unlikely to be segmented correctly. Any computer system that analyzes the lungs and does not work on manually delineated regions of interest must incorporate automatic lung segmentation.
The pulmonary segments are a reference system for radiologists, pulmonologists, and surgeons to indicate the position of lesions in the lungs. This allows a lobe based CT parameter extraction and thus a more accurate prediction of post operative lung function in case of a lobar resection, which is the standard treatment for early stage lung diseases. Automatic lung segmentation starts from different combined image segmentation and processing techniques. It starts with iterative thresholding and then enhances the fissure inside the lungs. We can use two different methods for lung segmentation one is edge tracking and another one is region filling.
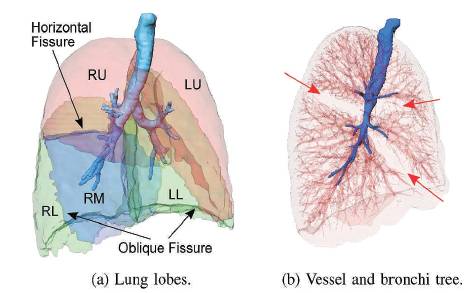
Figure 2. Lung lobes, vessels and bronchi tree
Based on the assumption that there are usually no major vessels at the lobar boundaries, the distance to the pulmonary vasculature is a suitable feature to detect lobar boundaries. To quantify the absence of vessels at the lobar boundaries, a coarse segmentation of the pulmonary vasculature is sufficient. There is high contrast between blood vessels and lung parenchyma that enables a coarse segmentation of the pulmonary blood vessels by thresholding the data inside the lung region. The goal is to include as many vessels as possible but exclude fissures and other dense structures. As the lung is essentially a bag of air in the body, it shows up as a dark region in CT scans. This contrast between lung and surrounding tissues forms the basis for the majority of the segmentation schemes. Segmentation is often a necessary first step to computer analysis. In CT of the lungs, the various anatomical entities that can require segmentation are the lungs themselves, the airways, the vessels and the lung lobes as in Figure 2.
The airways exhibit a tree structure (the trachea bronchial tree) of roughly cylindrical branches of decreasing radius in Figure 4. The trachea bifurcates into the left and right main bronchus. These bronchi repeatedly bifurcate (or trifurcate) into smaller bronchi. The bronchial lumen is (normally) filled with air, surrounded by the bronchial wall which has a relatively high CT value.
Each lung contains an arterial and a venous vessel tree. Where the pulmonary arteries and veins enter the lungs, their diameter can be up to 30 mm. As they branch, vessel diameters decrease. On a normal CT scan vessels can be seen up to 5–10 mm from the pleura. The arteries follow the course of the bronchial tree (when the bronchial wall is thickened, bronchus and artery have the appearance of a signet ring).
Figure 3 shows the pulmonary lobes from chest ct scans based on fissures, vessels, and bronchi.
To obtain a lobe segmentation from the cost image Down sampling of the cost image to a resolution of 1.5 mm x 1.5 mm x 1.5 mm is applied to reduce calculation time. The applied technique separates regions with local maxima in between and can be used with an arbitrary number of markers. The borders between the obtained lobes after the watershed segmentation are not always smooth due to local variations in the cost image. Two majority filters with different kernel sizes (3 x 3 x 3 and 5 x 5x 1) are applied in a row to smooth the boundaries. The label value that occurs most often under the kernel is set to the voxel. To obtain the lobe segmentation on the original resolution, the segmentation results are up sampled using nearest neighbour interpolation.
The threshold image contains fissures, other lung tissue structures and isolated voxels representing “noise”. A traditional image smoothing operation (e.g., a Gaussian filter) cannot be applied to the images because the fissures may be “smoothed out” together with image “noise”. To overcome this difficulty, we applied a statistical approach to extract the pulmonary fissures in threedimensional geometric space. This approach can distinguish between fissure surface (curved plane) and isolated small regions with random normal vectors (orientations).
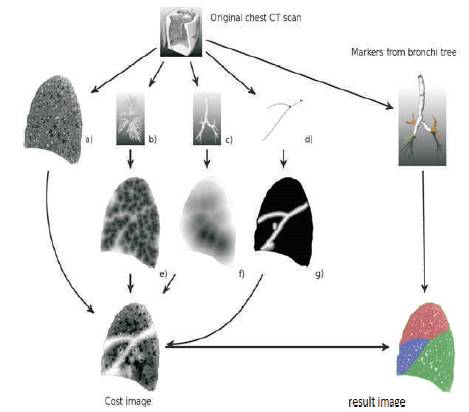
Figure 3. Automatic Lung segmentation
There are several different approaches of lung lobe and pulmonary fissure segmentation in literature. Binsheng Zhao [1] presents a computerized method for automated identification of small lung nodules on Multi Slice CT (MSCT) images. But by this method if a nodule is attached to a blood vessel and there is no density between the nodule and the vessel at their contact points, the nodule may not be able to be detected by this algorithm Bianca Lassen [2] gives a method in which a fully automatic lobe segmentation approach is presented, which is based on a previously proposed watershed transformation approach. This method increases segmentation accuracy where the fissures are visible. But the quality of lobe segmentation depends on a good bronchi and vessel tree segmentation. Shiying Hu [3] presents a fully automatic method for identifying the lungs in threedimensional (3-D) pulmonary X-ray CT images. E. M. van Rikxoort[4] presents fully automatic methods for segmentation of the lungs and lobes from thorax CT scans. The lung segmentation starts by automatically identifying the trachea and main bronchi. From the trachea, the lungs are found using a region growing approach. In cases for which errors are automatically detected in the resulting lung segmentation, a multi-atlas segmentation approach is applied. Multi-Slice CT scanning technology has revolutionized of the lungs and motivates the need for pulmonary image analysis. Segmentation of the lungs and lobes is a prerequisite for such image analysis in chest CT scans. Accurate lung segmentation allows for the detection and quantification of abnormalities within the lungs. Segmentation of the pulmonary lobes is important to localize parenchyma disease inside the lungs and to quantify the distribution of a parenchyma disease. This method is not able to include some severe abnormalities at the lung border.
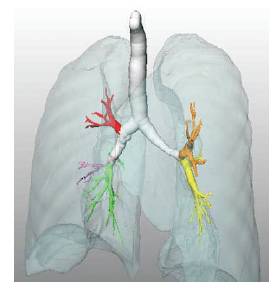
Figure 4. Analysed airway tree
Wook-Jin Choi and Tae-Sun Choi [5] proposed a novel pulmonar y nodule detection method based on hierarchical block classification. Z. Faizal Khan [6] proposed a method in which pre processing is done on the CT image of lung for removal of noise. A fuzzy filter is presented for the noise reduction of medical images corrupted with additive noise.
Medical imaging is the technique that is used to create images of the human body (or parts and function thereof) for clinical purposes (medical procedures seeking to reveal, diagnose or examine disease) or medical science (including the study of normal anatomy and physiology). The CT images offer detailed information of lung cavities, which could be used for better surgical planning of treating Lung Cancer. Pre processing is done to remove the noise from the isotropic CT image.
Filtering is the most fundamental operation in image processing and computer vision. The filtered image at a given location is a function of values of the input image in a small neighbourhood of the same location. Assuming that images vary slowly over space, near pixels is likely to have similar values. But this assumption fails at regions that contain edges and image details (e.g. corners, lines, end of lines etc.) Most of the classical linear filters like the averaging low pass filters tend to blur and destroy the lines, edges and other fine image details.
Jan-Martin Kuhnigk[7] proposed a method using an interactive 3D watershed algorithm which allows an iterative refinement of the results. This method requires large data set to process. Anita [8] developed a segmentation system in order to assist the surgeons to remove the portion of lung for the treatment of certain illness such as lung cancer, and tumours. The fissures of lung lobes are not seen by naked eyes in low dose CT image, there is a proposal for automatic segmentation system. The lung lobes and nodules in CT image are segmented using two stage approaches such as modified adaptive fissure sweep and adaptive thresholding. Initially pre-processing is used to remove the noise present in CT image using filter as shown in Figure 5 and Figure 6, then the fissure regions are located using adaptive fissure sweep technique, then histogram equalization and region growing is applied to refine the oblique fissure. Lung nodules are segmented using thresholding as in Figure 7.
A lobe segmentation method is developed which combines anatomical information from the lungs, vessels, airways, and lobar fissures to obtain the lobes using a Atlas method as in Figure 8. The approach is an extension of the framework of Bianca [9]. The method starts by segmenting the lungs, vessels, airways, and fissures, which are later combined into one cost image. In the first step lungs are segmented since all other segmentations are only performed inside the lung regions. A good lung segmentation is a prerequisite for the here presented lobe segmentation approach. The lung segmentation applied achieved the best performance in the LOLA11 [10] challenge.
Based on the assumption that there are usually no major vessels at the lobar boundaries, the distance to the pulmonary vasculature is a suitable feature to detect lobar boundaries. To quantify the absence of vessels at the lobar boundaries, a coarse segmentation of the pulmonary vasculature is sufficient. There is high contrast between blood vessels and lung parenchyma that enables a coarse segmentation of the pulmonary blood vessels by thresholding the data inside the lung region. The goal is to include as many vessels as possible but exclude fissures and other dense structures.
Since each lobe is separately supplied by subtrees of the bronchial tree, distance to the bronchi is a suitable feature to detect lobar boundaries, similar as for the vessels. In CT images, the airway lumen is dark and separated from the parenchymal tissue by thin airway wall structures that appear brighter. Segmentation of the airways in CT images is challenging because often the parenchymal fissures have similar HU values as the lumen, and both partial volume effect (PVE) and noise obscure the airway walls. We apply two preprocessing steps to mitigate these problems and to facilitate the segmentation with fixed kernel width (σ = 1.0 voxel) is applied to the image although the blurring increases the partial volume related problems. Second, a bronchi enhancement filtering is applied to the blurred image. Partial volume effects and the additional Gaussian blurring let the lumen of small airways appear brighter than normal air. The goal of the bronchi enhancement filtering is to detect voxels that are surrounded by dense circular structures as bronchi and to revert these volume averaging effects by decreasing their density again.
Figure 5. Input image
Figure 6. After the removal of noise
Figure 7. Segmented lungs
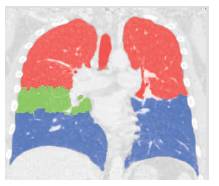
Figure 8. After the application of Atlas method final lungs, Fissures and lobe segmentation
Computed Tomography (CT) allows visualization of the lungs within a few seconds. Since typical scans with high anatomical details contain over 400 slices with sub millimetre resolution for each direction, manual segmentation is time consuming and there is demand for automatic lung lobe segmentation methods. The segmentation of pulmonary lobes is challenging because of anatomical variation and incomplete fissures. On the one hand, pathologies can deform the lobes and make the fissures unrecognizable. Water shed methods are generally time-consuming; segmentation of one case took 2 hour on average. Another disadvantage of this approach was that scans with lobar shapes not represented in the data set were unlikely to be segmented correctly. Any computer system that analyzes the lungs and does not work on manually delineated regions of interest must incorporate automatic lung segmentation.
A traditional image smoothing operation cannot be applied to the images because the fissures may be “smoothed out” together with image “noise”. To overcome this difficulty, we applied a statistical approach to extract the pulmonary fissures in three-dimensional geometric space. This approach can distinguish between fissure surface (curved plane) and isolated small regions with random normal vectors (orientations). Fissure enhancement consists of canny edge detection algorithm and followed by three morphological operations namely dilation, closing and thinning. The lobe segmentation can be obtained from the cost image. Down sampling of the cost image to a resolution of 1.5 mm x 1.5 mm x 1.5 mm is applied to reduce calculation time.
This method is generally time-consuming; segmentation of one case took 2 hour on average. To overcome this time delay a fully automated reliable and accurate results for low resolution CT images by atlas based lung segmentation method with minimum data set should be used and that should include the method for image retrieval system.