Table 1. Geological succession in and around Suran river, Poonch district, J&K State
Studies carried out on fifteen water samples of the Suran river from Bonikhet to Surankot, Poonch district, Jammu Himalaya in respect of Si4+, Ca2+, Mg2+, K+, Na+, Fe2+, Mn2+, Cu2+, Ni2+, Zn2 and Pb2+ reveal Fe2+, Mn2+ and Si4+ concentration above permissible limits and hence toxic for human consumption. However, no element among the chemically analysed was found harmful as far as agriculture is concerned. Turbidity values of Suran river water were found higher because of higher index of erosion in the watershed areas of Suran river. The different parameters for water, with respect to agricultural use namely: SAR (Sodium Adsorption Ratio), SSP (Sodium Soluble Percentage), RSC (Residual Sodium Carbonate), MR (Magnesium Ratio), CR (Corrosivity Ratio), EC (Electrical Conductivity), TDS (Total Dissolved Salts), TH (Total Hardness as CaCO3 are found all below permissible levels. TDS values are found < 500 ppm and hence suitable for domestic use.
The Suran river is the main river of the Poonch district. It is made up of number of streams flowing from the Pirpanjal and is joined by the subsequent streams from the Ratanpir (Figure 1). The main stream is Parnai which flows parallel to the Ratanpir and it takes its source at the bifurcation of Ratanpir from the main Pirpanjal. In the Parnai valley, in the higher reaches, there are number of lakes which also debouche its surface run-off into the Parnai nallah. Actually these lakes are situated in Panjal volcanics, south-east of Girjan valley (Fotedar et al., 1997). Out of these lakes, the highest altitude lake is Nandansar. This lake drains through Kashmir Himalaya and the rest of 7 lakes pour their run-off into Suran river through Parnai nallah. Parnai debouches its waters to Suran river beow Behramgala and has a source at a height above 3700 m. Exactly, the Suran river takes its source from the Pirpanjal Pass (Wakhloo and Shah, 1968; Fotedar et al., 1993; Fotedar et al., 1997). Suran stream is joined by a number of subsequent streams enroute to Chandimar, where it turns abruptly to south and is joined by another stream coming from north. The Suran after being joined by number of streams from the north forms a westerly course and is joined by Manai nallah from the east. The nallah makes a face of about 40 m and joins the Suran river. This fall is a historic Noorichamb fall supposed to have been favourite haunt of Noorjahan, the queen consort of the Moghul emperor Jahangir as they camped near Chandimar on their way to and from the Kashmir valley. The western course of the Suran river passes through Sailan, Bonikhet, Bafliaz, Draba, Fasalabad and finally into Surankot. All through, Suran flows through a gorge section which has a varying width from 100 m to 20 m. Other subsequent streams from the Ratanpir and the northern hills feed the main stream. At Bafliaz, the Suran river is joined by another mighty stream, the Chang nallah from the north, the stream takes its source from the foot of Tatakuti peak. The valley of Suran river widens considerably as it leaves gorge section at Bafliaz. Within its boulder bed the river divides into a number of branches which occasionally flow due south and are further joined by other ice-fed small streams.
There are about 140 villages situated in the vicinity of Suran river and the people of these villages use Suran water for drinking and other purposes. Present study is an attempt to make a detailed study in respect of water chemistry of Suran river for Municipal and domestic use.
The geology of the area is complex, firstly because it forms the farthest point in the North-West Himalayas and secondly because of the complex tectonic disturbance that the area has witnessed in the geological past due to uplift of Himalaya and the formation of syntaxial bend (Wadia, 1931; 1934) which culminates in Muzzafarabad (presently in Pakistan occupied Kashmir). The rocks are highly folded, flexured and over-turned and as such continuous succession is not discernible clearly except at Mandi, due to erosion and neotectonism. Various outcrops have been delineated by various workers from time to time (Wakhaloo and Shah, 1968; Srikantia, 1973; Rao et al., 1975; Karunakaran and Rangarao, 1979; Shah, 1980; Janpangi, 1986 and many other GSI scientists). Because many of litho units in the area are devoid of fossils, as such the age of the formations like Gamir, Baila and Mandi limestone are still debatable. However, the stratigraphic succession is worked out as done by earlier workers and our own observations in the field. The succession is given in Table 1.
For the present study, fifteen samples of Suran river (10 km water course) from Bonikhet to Surankot were collected in polythene bottles after measurement of pH on spot in the field. Trace element studies were carried out on Atomic Absorption Spectrophotometer Model 902, GBC Australian make, while other analysis was carried out by standard methods (Shapiro and Brannock, 1962). The chemical analysis of water samples and other quality parameters are listed in Tables 2 and 3. The water quality parameters were compared with the water quality standards for drinking and irrigation, chiefly with BIS (1991), WHO (1984), Kudesia (1983), Rodier (1975), DeWiest (1967) and Richards (1954). The techniques and method of collection and analysis of water samples followed in this study are those of APHA (1988) and CPCB (1997). The comparison of average values of different cations and other quality measurements are listed in Table 4.
Table 2. Chemical Analysis and other Quality Measurement Parameters for Waters of Suran river, Poonch district, J&K State
Table 4. Average concentration values of different cations, anions and values pertaining to other parameters for waters of Suran river, Poonch district, J&K State
Thin sections of the rocks slides, 60 in number of country rocks (containing schists, gneisses, slates, phyllites, traps, Baila-Gamirs and Zewan limestones) were made for detailed study to get insight into minerals contributing cations to the Suran waters. There exist no industries in the area and as such baseline data of ions contributed to Suran river by the minerals has been constructed. The modal mineralogy of the rocks reveals the cations, responsible for contaminating the waters.
The average concentration of calcium and magnesium in Suran river waters is 3.62 ppm and 1.08 ppm, respectively. The concentration of both these cations is safe to be used for human consumption (Table 4). These two cations are present too low in concentration these cannot be regarded harmful for agriculture.
Modal mineralogy of various rocks reveal Mg2+ ions contributed by biotites, pyroxenes of the volcanic which are abundantly present in Ratanpir, Girjan valley to the south-east of Bonikhet and to the north. The modal mineralogy of Salkhalas reveals hornblende schists, hornblende gneisses, amphibolites, sills and dykes in Salkhalas, together with tremolite-actinolite, tourmaline granites, chlorites, slates and dolomites, contributing in bulk the Ca2+ and Mg2+ ions to the waters. The close examination of Table 2 reveals Ca2+ and Mg2+ concentration falling abruptly low, when Suran river enters terrain of Murrees which constitute sands and clays in bulk. In sandstone terrains, calcium and magnesium gets leached downstream because of high porosity of sandstone grains. This behavior has well been observed in riverine-transport by sandstones by Wedepohl (1978), Dhar et al. (1996), Fotedar and Fotedar (2009b). Magnesium has more replacing action in the carbonate system of rocks better than potassium, sodium and calcium and as such which magnesium replacing calcium in the formatioin of dolomites (existing in dolomitic horizons of Salkhalas) dissolves more quickly in the system (Goldschemidt, 1954; Engel et al., 1964). This is the reason that concentration of magnesium in waters of Suran river is lower than that of calcium in all the 15 water samples. The depletion of magnesium in Suran waters may also be due to formation of chlorite mineral present in slates and phyllites during late diagenesis (Deer et al., 1961; Von Engel Hardt and Gaida, 1963; Fotedar and Fotedar, 2009d).
Calcium and magnesium are both utilized for calculation of Magnesium Ratio (MR) expressed as:
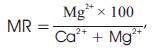
where all the ions are expressed in epm
According to Aravindan (2004), MR should be less than 50, which is considered good for crops. In case of Suran river waters, the values for all 15 water samples is less than 50 and as such with respect to this value, the waters of Suran river can safely be used for agricultural purposes.
Calcium and magnesium are also utilized for calculating total hardness as CaCO3 by the formula proposed by Raghunath (1987).
Total Hardness as CaCO3 = (Ca2+ + Mg2+) meq × 50
The total hardness values for Suran river water lie in between 13-20 ppm, indicating these values to fall under “safe category”, so the waters can be used for human consumption and for agriculture safely (Table 4).
Iron (Fe2+) concentration in Suran river waters ranges between 0.31 ppm to 0.77 ppm with an average value of 0.48 ppm. Table 2 reveals all values of Fe2+ present above permissible limits and hence the waters are toxic for domestic use. However, Fe2+ concentration is within safer limits as far as agriculture is concerned (Table-4). Fe2+ and Fe3+ appears to have been contributed by haematitic phyllites, slates, schists, gneisses, ferruginous limestones of Salkhala Formations; bulk of iron contained by Zewan Formation occurring in northern watershed of Suran river. Besides, bulk of iron appears to have been contributed by Gamir and Baila Formations, and Meta volcanic occurring in Pirpanjals, Ratanpir hills and volcanic horizons occurring in Girjan valley traps around lakes. The modal mineralogy of Girjan valley volcanic worked out by Fotedar et al. (1997) and revised presently is as under:- pyroxene = 7.00%, epidote-zoisites = 16.03%, opaques including iron sulphides, copper-iron sulphides etc. = 5%, actinolite-tremolite = 4.06%, iron in calcites = 200%, chlorites = 14.05%, totalling = 41.14%. Together with Baila-Gamir constitute iron minerals (5%) and iron-bearing minerals of Salkhalas including mica-schists, chlorite schists, slamolite-schists, chlorite-phyllitic schists (15%), the sum total of modal mineralogy comes to be 61.14%.
Iron bearing minerals present in the watershed of Suran river apparently reveals that Salkhalas, Zewans, Girjan valley meta-volcanics, Ratanpir volcanic and Panjal volcanic contribute iron cations heavily to the waters revealing the reason for high content of Fe2+ in the Suran waters. Due to erosion, a lot of clay and organic matter from Salkhalas containing adsorbed iron also has played a major part in contributing iron to the waters (Horowitz, 1974; Gibbs, 1977; Jenne et al., 1980; Fotedar et al., 2008a; Fotedar et al., 2008b; Fotedar and Fotedar, 2009a; 2009b; 2009c; Fotedar et al., 2009).
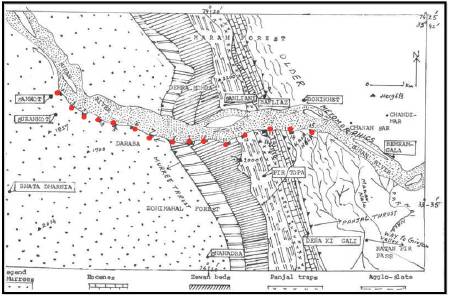
Figure 1. Map of Suram River between surankot to Bonikhet, Poonch Area (After Rao et. Al. and personal observation of the authors in the field
Mn2+ concentration in waters of Suran river ranges between 0.06 to 0.24 ppm with an average value of 0.14 ppm. The Mn2+ concentration of potable water should be < 0.1 (Wedepohl, 1978), but the values for all samples have crossed safer level value and hence Mn2+ too like Fe2+ is toxic for domestic use (Table 4). Manganese is geochemically associated with iron and it is also contained by the mineral cited in Table 4, which can be said to have contaminated Suran waters with this cation and manganese also like iron gets absorbed in fine clays and organic matter during riverine transport. Mn2+ concentration of Suran river waters however is not harmful for agricultural use (Table 4).
Na+ and K+ both occur in low concentrations in Suran river water. The contributing minerals for these cations are feldspars. The modal mineralogy of various rock studies of the area has revealed that modal feldspars have declined enormously from the rocks till Suran river crosses sandstone terrain of Murree outcrop. These two cations with low concentrations cannot be said to be toxic for domestic use or agricultural use.
SAR is less than 6 and SSP is less than 60, are good for agricultural use (Table 4).
RSC for all the 15 water samples is observed to be less than 1.25, hence Suran waters are safe to be used for agricultural purposes as far as this parameter is concerned (Table 4).
pH of all the samples is within the prescribed limits (6.5-8.5) normally acceptable as per guidelines suggested by WHO (1984).
HCO3–, SO42–, Cl– and NO3– are present in safer limits and cannot be considered harmful for domestic use and agricultural use (Table 4).
The range of Si4+ in the water samples lies between 26 ppm to 38.08 ppm with an average of 30.45 ppm. More silica present in Suran waters reflects high index of weathering witnessed by the rocks of the area due to erosion. This has caused high turbidity in waters. The turbidity increases manifold during monsoons. The permissible limit for silica in drinking water is 15 ppm (BIS, 1991) and all the samples have silica concentration more than this which should be taken as objectionable. The waters need sufficient filtration before use for human consumption.
All these cations are observed to be present below permissible limits in Suran waters (Table 4). Therefore, with respect to these, the waters are not harmful if used for both Municipal and agricultural uses.
The range of TDS lies between 46-60 ppm. The permissible limit of TDS for potable waters is 500 ppm, but in the present case, the values of Suran river waters are far less than this value. Hence, with respect to this parameter, the waters are safe to be used for domestic use. For agricultural purpose also, the values are safer to be used with respect to this parameter (Table 4).
The EC values for Suran river waters in micromhos/cm at 250C ranged between 0.037-0.051. According to Richards (1954) and US Laboratory Salinity Staff (1984), the values fall in “low salinity zone” and hence the waters can safely be used for irrigation on all types of soils.
The corrosivity ratio (CR) in case of Suran river waters has been calculated for all the 15 samples. The corrosive ratio is defined by the formula:
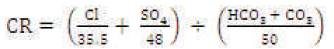
where all the ions are expressed as ratio of alkaline earths to saline salts in water (Aravindan et al., 2004). The values < 1 are considered safe and in the present case, the values of all fifteen samples lie between 0.55-0.75. Therefore, Suran river waters are safe to be used for transportation of water through metallic pipes in the agricultural fields without causing corrosion.
1.In order to check erosion, side repose slopes should be carved out on hills in the north and south. On these slopes with modern improved silvicultural methods, contour farming can be initiated.
2.Forest in the watersheds of Suran river have completely been removed by the local people. There is lot of erosion occurring on the hill slopes. For checking erosion, the watershed in north and south should be provided with adequate forest cover. This will arrest many of unwanted inorganic and organic materials to pass into solution of waters. Also on both the river banks of Suran river from Bonikhet to Surankot, vetiver grass should be grown. It will efficiently check unwanted ions to move into the water especially Fe2+, Mn2+, Si4+ which are present above the permissible limits in Suran river. Vetiver grass plantation is a tested technology having been used in Himalayan terrains for checking mass wastage and contamination of water (Lavania, 2004).
3.Suran valley has enchanting hill topography on its north and south, the whole basin has the scope of adding to its banks, parks and terraced gardens which are bound to attract tourists from all over India.
4.There is no Municipal filtration plant in the area and as such a big tank at Bonikhet or Bafliaz may be constructed to filter the waters, together with other hygienic treatments carried out before supply of water to different villages for the whole population could be made through non-corrosive water pipes. The hygiene treatment of water will keep many waterborne diseases away that otherwise affecte very year a large chunk of population in this remote areas of Poonch district.
5.In order to control erosion, the watershed of Suran river in the north and south needs adequate afforestation. Vetiver grass technology on the intermediate heights of the hills should be started forthwith to curb landslides, mass wastage, and it will also arrest unwanted cations to enter into the solution of waters of Suran river. Vetiver technology is a tested technology have been used in many Himalayan terrains for checking contaminants to run into water bodies besides controlling mass wastage and landslides.
6.There is ample scope of constructing parks on side-repose-slopes near to both banks of Suran river, this will improve the whole area for ecotourism.
7.A filtration tank should be constructed at Bonikhet or Bafliaz for usual hygiene treatment of water together with arrangements for disinfection with chlorine/ bromine before supplies could be made to whole population through pipelines.