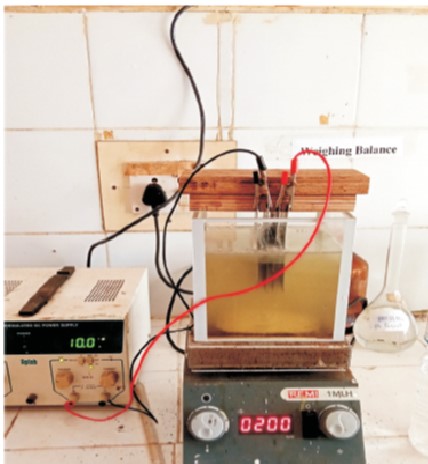
Figure 1. Electrocoagulation Setup
Municipal wastewater can include many pollutants that have an effect on the environment, so treatment is very necessary before discharge into water bodies and for further recycling in recreation and agriculture. As a result, in this study, municipal wastewater is treated using Electro Coagulation (EC) and Chemical Coagulation (CC), with comparisons of both processes. For EC, aluminum electrodes and iron electrodes were used at different voltages and times. Aluminum sulphate and ferric chloride were used in the CC, along with coagulant dosage and contact time. After treating the municipal wastewater for electrocoagulation, the maximum removal efficiency of COD, TOC, TDS, and BOD is 85%, 87%, 82%, and 81% for the aluminum electrode, respectively and 92%, 92%, 84%, and 88% for the iron electrode, respectively. For chemical coagulation, the maximum removal efficiency of COD, TOC, TDS, and BOD is 81.66%, 80.09%, 84.67%, and 77.08% for aluminum sulphate as a coagulant, and 86%, 83.90%, 87.73%, and 81.8% for ferric chloride as a coagulant. Electrocoagulation was found to be superior to chemical coagulation in this study. And Fe is a very promising electrode compared to Al.
The most pressing issue with water today is supplying an ever-increasing population in the twenty-first century. As a result, whether the goal is to discharge water into the environment or recycle it, humankind must maintain and manage water resources. The advanced treatment technology is investigated by water resource management because it requires the removal of pollutants from inadequately treated effluent by the treatment plants (Tchamango et al., 2017). In this present work, a comparative study of electrocoagulation and chemical coagulation is represented as a treatment process for municipal wastewater discharges from municipal treatment plants (Andrade, 2009). This comparison is about Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD), Total Dissolved Solids (TDS), and Total Organic Carbon (TOC).
The objectives of this paper are to study batch electrocoagulation using aluminum and iron electrodes for the removal of pollutants such as COD, TOC, TDS, and BOD; to study batch chemical coagulation using aluminum sulphate and ferric chloride as coagulants for the removal of COD, TOC, TDS, and BOD, and to compare both electrocoagulation and chemical coagulation by pollutant removal efficiency.
Municipal wastewater is one of the most significant environmental pollutants, consisting of liquid wastes emanating from residential, commercial, or institutional buildings, such as latrines, urinals, bathrooms, kitchen sinks, washbasins, and so on, and containing contaminants such as heavy metals, total suspended solids, BOD, COD, and so on. The effluents from industrials contain a high concentration of contaminant pollutant, which when discharged directly into a river causes major problems and serious environmental damage.
The primary goal of wastewater treatment is to remove contaminants in an efficient and cost-effective manner (Zaleschi et al., 2012). The treatment of municipal wastewater by electrocoagulation is a cost-effective technique for treating effluent with no addition of chemicals. It also decreases the amount of sludge (Tlaiaa et al., 2020). The electrocoagulation process may reflect an advanced oxidation process and be used in wastewater treatment (Tchamango et al., 2017). It involves the causation of coagulants by electrodissolution of sacrificial anodes, generally aluminum and iron electrodes. Chemical coagulation treatment is also a cost-effective and environmentally friendly method for this treatment. The addition of coagulants like aluminum sulphate and ferric chloride requires the negative charge of suspended particles over neutralization; the suspended particles stick with each other to form larger particles for efficient coagulation, and rapid mixing is more effective (Ukiwe et al., 2014).
Ozturk and Özcan (2021) investigate the efficacy of coagulation methods using Al electrodes and Al2 (SO4)3.18H2O on cardboard manufacturing wastewaters. In the electrocoagulation experiments, the optimum current and pH where the highest COD removal efficiency of 54% was achieved were determined, respectively, as 10 A and 6.62. It was also discovered in the study that a KCL concentration of 0.5 mg/L achieved 58% COD removal.
Verma and Kumar (2018) analyze landfill leachate and municipal wastewater at various ratios (1:20, 1:10, 1:7 and 1:5) which were subjected to coagulation and Electro Coagulation (EC). Alum was used in conventional coagulation at pH 6 and aluminum plate as electrode was used in EC at a current density of 386 A/m2 with 5 cm inter electrode spacing. Treatment efficiency was assessed from removal of Chemical Oxygen Demand (COD), Total Suspended Solids (TSS), turbidity, ammonia, nitrate and phosphate.
Tran et al. (2012) produced an effluent suitable for stream discharge, and electrochemical techniques have been explored at the laboratory pilot scale for total phosphorus (Ptot) removal from spiked Municipal Waste Water (MWW). The MWW was an effluent provided by a bio-filtration process installed at the Quebec Urban Community Water Treatment Plant (WTP). The effects of current density, retention time, and initial Ptot concentration were tot investigated using a bipolar electrolytic cell made of mild steel electrodes.
Akbal and Camci (2010) depict chemical coagulation as being performed using either aluminum sulphate or ferric chloride, whereas electrocoagulation was done in an electrolytic cell using aluminum or iron electrodes. By chemical coagulation, Cu-, Cr-, and Ni- removal of 99.9% was achieved with aluminum sulphate and ferric chloride dosages of 500, 1000, and 2000 mg L1, respectively. The removal of metals by electrocoagulation was affected by the electrode material, wastewater pH, current density, number of electrodes, and electrocoagulation time.
Arambarri et al. (2019) mentions combining sequential Electro Coagulation (EC) and Chemical Coagulation Treatment (CC) processes. Synthetic and microbrewery wastewaters were tested in this investigation. The generated results demonstrated the capacity for the integration of EC-CC to effectively remove phosphorus contamination from wastewater under varying operating conditions. The effects of several operational parameters, such as current density, conductivity, nutrient loading, and electrolysis time, were investigated. The results showed that increased salinity can significantly accelerate the removal of phosphorous during EC treatment, with 84.2% and 92.4% removal found for the applied powers of 5 and 10W, respectively.
Electrocoagulation is a complex process with many synergistic mechanisms operating to remove water pollutants. Electrocoagulation is a process that simultaneously removes heavy metal ions, solids in suspension, organic emulsions, and many other water pollutants using electric energy and sacrificial metallic plates instead of expensive chemical reagents. Chemical Coagulation: Flocculation is a physical chemical process that is commonly used in water purification and wastewater treatment to remove colloids. Chemical species' actions destabilize colloidal particles, which then aggregate into large aggregates.
Municipal wastewater was collected from the sewage treatment plant at Navanagar in Bagalkote. The acrylic glass sheet reactor for electrocoagulation has dimensions of 15cm×15cm×15cm, the four aluminum and iron electrodes with mono-polar parallel connection have dimensions of 10cm×10cm×0.1cm and the distance between each electrode is 10 cm, and the DC power supply of the applied current was 5 v to 20 v with time intervals ranging from 15 min to 60 min, and the magnetic stirrer has a speed of 200 rpm (Emara et al., 2018).
An alum jar test apparatus with six beakers, a stirring paddle at 200 rpm aluminum sulfate Al2 (SO4)3, and Ferric Chloride (FeCl3) with coagulant dosage of 3ml/L to 15ml/L, 3 and a weighing balance are required for chemical coagulation (Padmaja et al., 2020).
An electrocoagulation experiment is carried out in a batch reactor that was designed and fabricated to treat municipal wastewater (Cañizares et al., 2009; Mollah et al., 2004). The batch reactor is made of acrylic glass sheet and has dimensions of 15cm×15cm×15mm a thickness of 5 mm, and a volume of 2 L. Then, 2L of wastewater was poured into the reactor with a magnetic stirring bar and kept on a magnetic stirrer, and four aluminum electrodes with a mono-polar parallel connection were immersed in the wastewater, with dimensions of 10 cm x 10 cm x 1 mm and a thickness of 1 cm. A magnetic stirrer was used for homogeneous mixing of the solution with a magnetic string bar. to the DC power supply, and voltages are set at 5 v, 10 v, 15 v, and 20 v and different time intervals of 15 min, 30 min, 45 min, and 60 min at 200 rpm. After the treatment, the COD, BOD, TDS, and TOC were determined. The procedure was then repeated with four iron electrodes of the same size (Tchamango et al., 2016). Figure 1 shows the electrocoagulation setup used for the electrocoagulation process.

Figure 1. Electrocoagulation Setup
The main reaction of chemicals appearing on electrodes during electrolysis is the disintegration of metal cations at the anode, followed by the generation of hydroxyl ions and H gas at the cathode, as shown below (Alshawabkeh 2 et al., 2004).
At anode: Ms → M(aq)n+ + ne-
2H2O(l) → 4H(aq) + O2(g) +4e-
At cathode: M(aq)n+ + ne- → Ms
2H2O(l) + 2e- → H2(g) + 2OH-
In case that aluminum or iron electrodes are conducted, the developed Al(aq)3+ or Fe(aq)3+ ions will rapidly have another reaction to create interrelated hydroxides (Bechtold et al., 2002). For example, ions of Al3+ in hydrolysis possibly will make Al(H2O)63+, Al(H2O5)OH2+, Al(H2O4)(OH)2+, a lot of monomeric and polymeric group formed by hydrolysis products such as Al(OH)2+, Al2(OH)24+, Al(OH)4, Al6(OH)153+, Al8(OH)204+ , Al13(OH) , Al3+O4 (OH)247+, Al13(OH)345+ in wide range of pH. Correspondingly, ferric 13 34 ions are caused through electro chemical decomposition of monomeric ions formed by iron electrode, Fe(OH)3 and hydroxyl compound, such as Fe(H2O)63+, Fe(H2O)6 (OH) , Fe2(H2O)8 (OH)24+, Fe2(H2O)8 (OH)24+ and Fe2(H2O)6 (OH)44+. The arrangement of these compounds depend fully on the solution of pH (Hussein & Jasim, 2021).
As shown in Figure 2, this process has three stages. The first stage represents the coagulation. Here in this stage, wastewater is treated with the addition of coagulants like ferric chloride and aluminum sulphate with rapid mixing, then customized by converting the wastewater's negative charge particles into positive charge particles; in this way, the particles tend to form larger aggregates (Bensadok et al., 2008). In the second stage, a slow stirring process is performed, and flocculation occurs based on the aggregation of colloidal particles, which destabilized the formation of large colloidal particles in the first stage to allow sedimentation. Due to the addition of polyelectrolyte, flocks are formed. Because of the flocculation phenomenon associated with large particles that settle easily, the polymeric organic molecules are ionized between the particles. In the final stage, the flocks formed in the settling unit are separated from the treated wastewater to produce sludge (Assadi et al., 2016). A flow chart of the step-by-step process of coagulation is shown in Figure 2.
A chemical coagulation experiment was carried out using a jar test apparatus. The jar test apparatus consists of five 1 liter beakers and a stirring device that homogeneously mixes the solution (Assadi et al., 2016). The municipal wastewater is measured to 1 liter in all beakers, the wastewater sample jar is fixed to the stirring devices, and the paddles are moved in an upward manner at the same heights. The coagulant of Alum is added with a dosage of 3 ml, 6 ml, 9 ml, 12 ml, and 15 ml with different time intervals of 15 minutes to 60 minutes with an automatically operated paddle at 200 rpm. After the stirring is finished, the jar and stirring tool are removed and set aside for 30 minutes to allow the flocks to develop and settle. The samples are collected without disturbing the sediment, and the COD, BOD, TDS, and TOC of the sample are determined. The same procedure was done with ferric chloride. Then the two treatments are compared, electrocoagulation and chemical coagulation, with the removal efficiencies of COD, BOD, TDS, and TOC. Figure 3 shows the chemical coagulation setup for the chemical coagulation process.

Figure 3. Chemical Coagulation Setup
In this portion the wastewater treated by electrocoagulation with aluminum electrodes, wastewater treated by electrocoagulation with iron electrode, wastewater treatment by chemical coagulation with aluminum sulfate as wastewater treatment by chemical coagulation with ferric chloride as coagulant, and comparison of electrocoagulation and chemical coagulation with the results and brief discussion of these are presented.
Electrocoagulation of municipal wastewater was carried out using electrodes in a batch reactor with a mono-polar parallel connection. The output graphs for the wastewater treated by electrocoagulation with aluminum electrodes for various parameters, which are plotted against time, are depicted in Figure 4.
Figure 4. (a) COD Removal Efficiency (b) TOC Removal Efficiency (c) TDS Removal Efficiency (d) BOD Removal Efficiency With Different Voltages and Contact Time for Electrocoagulation with Aluminum Electrode
Municipal wastewater treatment by electrocoagulation was carried out in a batch reactor with a mono-polar parallel connection of four iron electrodes used at different voltages and contact times depending on the number of experiments carried out (Hussein & Jasim, 2021). Figure 5 depicts the output graphs for the wastewater treated by electrocoagulation with COD removal efficiency, TOC removal efficiency, TDS removal efficiency, and BOD removal efficiency with different voltages and contact times for electrocoagulation with an iron electrode.
Figure 5. (a) COD Removal Efficiency (b) TOC Removal Efficiency (c) TDS Removal Efficiency (d) BOD Removal Efficiency With Different Percentages and Contact Time Electrocoagulation with Iron Electrode
The treatment of municipal wastewater by chemical coagulation was prescribed using a jar test device with Aluminum Sulfate (Al2(SO4)3) as a coagulant and different 2 4 3 coagulant dosages and contact times. The output graphs for the wastewater treated by chemical coagulation with COD removal efficiency, TOC removal efficiency, TDS removal efficiency, and BOD removal efficiency with different voltages and contact times for chemical coagulation with aluminum sulphate are shown in Figure 6.
Figure 6. (a) COD Removal Efficiency (b) TOC Removal Efficiency (c) TDS Removal Efficiency (d) BOD Removal Efficiency with Removal Percentage and Coagulant Dosage for Chemical Coagulation with Aluminum Sulfate
Treatment of municipal wastewater by chemical coagulation was carried out using jar test equipment with Ferric Chloride (FeCl ) used as a coagulant with different 3 coagulant dosages and contact times. The graphs for the wastewater treated by chemical coagulation with COD removal efficiency, TOC removal efficiency, TDS removal efficiency, and BOD removal efficiency with different voltages and contact times for chemical coagulation with aluminum sulphate are shown in Figure 7.
Figure 7. (a) COD Removal Efficiency (b) TOC Removal Efficiency (c) TDS Removal Efficiency (d) BOD Removal Efficiency with Removal Percentage and Coagulant Dosage for Chemical Coagulation with Ferric Chloride
After conducting an experiment of electrocoagulation and chemical coagulation with Al and Fe, the results of COD, TOC, TDS, and BOD were determined and compared. The main comparisons are EC with an aluminum electrode, EC with an iron electrode, CC with coagulant aluminum sulfate, and CC with coagulant ferric chloride. The graphs for the same are depicted in Figure 8.
Figure 8. (a) EC With Aluminium Electrode (b) EC with Iron Electrode (c) CC with Coagulant Aluminum Sulfate (d) CC with Coagulant of Ferric Chloride with Different Voltages and Contact Time Shows the Comparison of Electrocoagulation and Chemical Coagulation
From the comparison of both the electrocoagulation and chemical coagulation, it can be found out that initially, for EC with an aluminum electrode, the TOC is comparatively higher compared to the other three when time is a major factor. Considering EC with an iron electrode, COD is a relatively higher percentage compared to other parameters as far as time is concerned. TOC is a higher percentage of the remaining parameters in CC with coagulant aluminum sulphate than in CC without coagulant. Finally, for the consideration of CC with coagulant ferric chloride, the higher values are similar to those for CC with coagulant aluminum sulphate.
According to the findings of the investigation, increasing electrical current and coagulant dosage increased the maximum removal efficiency of COD, TOC, TDS, and BOD. For the aluminum electrode, the maximum removal efficiency of COD, TOC, TDS, and BOD is 85%, 87%, 82%, and 81%, respectively. 92%, 92%, 84%, and 88% for the iron electrode, respectively. For chemical coagulation, the maximum removal efficiency of COD, TOC, TDS, and BOD is 81.66%, 80.09%, 84.67%, and 77.08% for aluminum sulphate as a coagulant, and 86%, 83.90%, 87.73%, and 81.8% for ferric chloride as a coagulant. Comparison of EC and CC by Al and Fe: Fe has maximum removal efficiency compared to Al. And in the relationship of EC and CC with the removal efficiency of COD, TOC, TDS, and BOD, electrocoagulation achieved maximum removal efficiency, so that electrocoagulation is the best technology for wastewater treatment compared to chemical coagulation, and also Fe is a very promising electrode compared to Al.