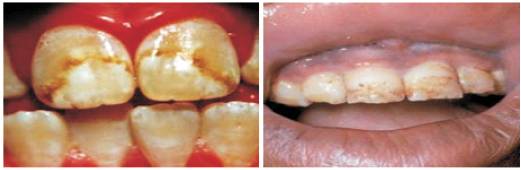
Figure1. Fluorosis due to high amount of Fluoride
The main objective of the experimental investigation was to develop inexpensive and safe methods of fluoride removal. The adsorbents used in this investigation are amla powder, tamarind powder, neem powder; brick powders, clay with sand which were inexpensive and nontoxic materials available easily in nature and locally at village/rural level. Some of the adsorbents are very effective in removal of fluoride ions,and can be used as fluoridating agents but they impart color and turbidity to the treated drinking water. Among all adsorbents, amla powder was found to be the most cost effective in adsorbing fluorine ions. Tamarind and brick powder were also found to be efficient adsorbents to certain extent. Clay mixed with different percentage of sand was also capable of adsorbing fluoride ions at lower pH value. Both batch and column experiments were conducted, and fluoride removal capacity was studied with respect to time and flow rates lit/day respectively. Finally, it has been observed that adsorbents were definitely having good adsorbent capacity, pH and fluoride concentration and are well within permissible limits.
Pure water is scarce and is not easily available to all. The water may be contaminated by natural sources or by industrial effluents. One such contaminant is fluoride. Geological formation is the main source of fluoride in groundwater. Fluoride is a naturally occurring compound derived from fluorine, the 13th most abundant element on Earth. It is found in rocks, soil, and fresh and ocean water. Fluoride occurs naturally in public water systems as a result of runoff from weathering of fluoride-containing rocks and soils and leaching from soil into groundwater. Atmospheric deposition of fluoride-containing emissions from coal-fired power plants and other industrial sources also contribute to amounts found in water, either by direct deposition or by deposition to soil and subsequent runoff into water [6].
Fluorine is beneficial to human health, if its concentration is within permissible limits of 1mg/l. But, on the other hand, if it is within 1mg/l, it helps human body in many ways like growth of teeth and enamel from dissolving in acidic conditions [1,2]. If its concentration exceeds 1mg/l, a disease called fluorosis occurs which is incurable and effects caused by them is also detrimental. If the concentration of fluoride rises from 1.5mg/l-2mg/l, then dental fluorosis occurs [1,2]. Sea water, processed foods made with fluoridated water, fluoride-containing pesticides, bottled teas, fluorinated pharmaceuticals, teflon pans, and mechanically deboned chicken are the main sources of fluoride causes [3,4] . Various dietary components influence the absorption of fluorides from gastrointestinal tract and the absorbed fluoride are distributed throughout the body [4,5]. A higher percentage of fluoride may also cause serious health hazards. The diseases [Figures 1 & 2] caused manifests in three forms; dental, skeletal and non- skeletal fluorosis [3,5].

Figure1. Fluorosis due to high amount of Fluoride

Figure 2. Skeletal Fluorosis due to high amount of Nitrates
The major sources of fluoride in ground water are fluoride bearing rocks such as fluorspar, cryolite, fluorapatite and hydroxylapatite. The fluoride content in the ground water is a function of many factors such as availability and solubility of fluoride minerals, velocity of flowing water, pH, temperature, and concentration of calcium and bicarbonate ions in water [8]. The other sources of fluoride occurrence in water are industrial discharge from aluminum industries, phosphate industries, coal plants as well as due to water, food, air, medicament and cosmetics [10].
Several sophisticated methods and ways have been suggested and practiced for fluoride removal in drinking water. Those methods are expensive and sophisticated devices which cannot be afforded by people in village/rural areas [9].
The main objective of this experimental investigation was to develop inexpensive and safe methods of fluoride removal. The adsorbents used in this investigation are amla powder, tamarind powder, neem powder; brick powders, clay with sand [7], which are inexpensive and nontoxic materials available easily in nature and locally at village/rural level. Some adsorbents are very effective in removal of fluoride ions and can be used as fluoridating agents but they impart color and turbidity to the treated drinking water. Among all adsorbents; amla powder was found to be most cost effective in adsorbing fluorine ions. Tamarind and brick powder were also found to be efficient adsorbents to certain extent. Clay mixed with different percentage of sand was also capable of adsorbing fluoride ions at lower pH value. Both batch and column experiments are conducted, and fluoride removal capacity was studied with respect to time and flow rates lit/day respectively. Finally, it has been observed that adsorbents were definitely having good adsorbent capacity, and pH and fluoride concentration are well within permissible limits.
The main objective of this experimental work was to
The aim of the experimental work was to develop an inexpensive and safe method of fluoride removal.
Amla: It is sour, bitter and rich in vitamin C and it is quantified as four times more than orange, eight times more than a tomato and ten times more than apple. Intake of 50 ml amla juice (1.5 spoon) every day meets the daily requirement of vitamin C of human body. It is also having gallic acid, tanic acid, albumin, calcium, protein, phosphorus, carbohydrate, iron etc. Amla has many curative properties, it controls imbalance caused by various reactions occurring in our body, and can be used as general tonic for weakness, eye ailments, mental weakness, to strengthen cardiac muscles, acidity, digestive problems, diarrhea, dysentery, constipation, impart natural glow to hair and provide luster to body. Totally it's a wonderful material available in nature.
Tamarind: Tamarind is one of the vegetables/fruits having very good commercial value and available commonly in India and Africa. Manufactured products available in market are tartaric acid, invert sugars, food color, crude pectin, tamarind protein and tamarind seed oil. It is also used in pharmaceutical industries, cleaning chemicals in boilers and heat exchanger, for the preparation of jellies, jam, marmalade, bubble gum etc. Tartaric acid is one of the import substitute high value product and used largely in industries; and in food items, for the preparation of different synthetic chemical products, paint industry, soap industry etc.
Neem powder: All parts of the tree (seeds, leaves, flowers and bark) are used for preparing many different medical preparations. Neem oil is used for preparing cosmetics (soap, shampoo, balms and creams), and is useful for skin care such as acne treatment, skin elasticity. Neem oil has been found to be an effective mosquito repellent. Neem derivates neutralize nearly 500 pests worldwide, including insects, mites, ticks and nematodes. Neem does not normally kill pests right away; rather it repels them and affects their growth. As neem products are cheap and non-toxic to higher animals and most beneficial insects, it is well- suited for pest control in rural areas.
Brick: A brick is a block or a single unit of a kneaded clay-bearing soil, sand and lime, fire hardened or air dried, used in masonry construction laid using mortar. It has relatively high compressive strength but significantly low tensile strength. Researches have been made in fields of deflouridation of drinking water and it is attempted to show that brick can also be used as a material that adsorbs fluoride onto its surface.
Clay and sand: Clay is often referred to as the alumino silicates formed from chemical weathering. It also contains hydrated oxides of ions of aluminum and manganese. The particles in the clay fraction range from crystalline through poorly crystalline to amorphous as detected by X-ray diffraction.
All reagents used were of A R grade. Stock fluoride solution was prepared by dissolving 221mg of sodium fluoride in distilled water and diluting to 1000ml. Then standard fluoride solutions were prepared by diluting 100ml stock fluoride solution to 1000ml of distilled water.
SPADN Solution: 958 mg of SPADN was dissolved in distilled water and dilution was one upto 500ml. This solution is stable for at least one year, if protected from direct sunlight. The calibration curve obtained from SPADN method is shown in Figure 3.
Zirconyl acid reagent: 133 mg of zirconyl chloride octahydrate was dissolved in about 25ml of distilled water, 350ml of conc. HCL was added and diluted to about 500ml of distilled water.
Acid zirconyl–SPADN reagent solution: Equal volumes of SPADN and zirconyl acid reagent are mixed and stored. This solution is stable for at least 2 years, if protected from sunlight.
Reference solution: 10 ml of SPADN solution was added to 100 ml distilled water. 7 ml of conc. HCL was diluted to 10ml and then added to SPADN solution.
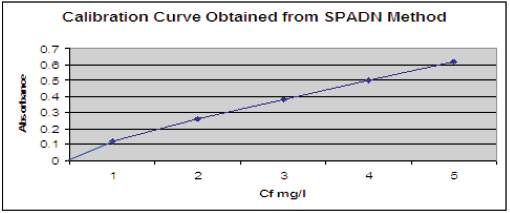
Figure 3. The calibration curve obtained from SPADN method depicted in the form of graph.
The resulting solution is used for setting the instrument to zero reference point and this solution is also stable for one year.
Preparation of standard curve: Fluoride standards were prepared in the range of 0 to 1.4mg F/l by diluting appropriate quantities of standard fluoride solution to 50 ml with distilled water 0.5ml each of SPADN solution and zirconyl acid reagent was added to each standard and mixed well. Contamination was avoided. The photometer was set to zero absorbance with the reference solution and absorbance readings of standards were obtained (Figure 4). A curve of milligrams fluoride- absorbance relationship was plotted. A new standard curve was prepared whenever a fresh reagent is made. Here, absorbance was measured at 570 ml fluoride concentration in the water sample.
Sample pretreatment: If the sample contains residual chlorine, the same was removed by adding one drop of sodium arsenite solution or by adding 0.1mg residual chlorine and mixed well.
Color development: A 50ml sample or a portion of the sample was used and diluted with distilled water. Sample temperatures are adjusted to that used for standard curve. 10 ml of mixed SPADN-zirconyl acid reagent was added and absorbance was measured, first setting the reference point of the photometer. If the absorbance falls beyond the range of the standard curve, the trial was repeated using a diluted sample.

Figure 4. UV Spectrophotometer
Fresh neem leaves were dried in an oven for 6-8 hrs. The leaves were crushed in a grinder to get a fine powder of grain size 1.6-1.8mm. Tamarind was got from market in the form of fruit and dry powder of grain size 1.6-1.8mm. Amla was also got from market in the form of dry powder and it was made to undergo sieve analysis to obtain the grain size of 1.6-1.8mm. Brick was crushed in a crusher to get uniform powder of the same grain size.
The experimental setup for batch experiment is shown in Figure 5. A known mass of adsorbent powder was added to 50ml of water in a glass beaker. The initial concentration of fluoride in water (Cf) was 2.5mg/l. The mixture was stirred using a magnetic stirrer and fluoride concentration was measured at different time intervals using SPADN method, along with pH value. The experiment was repeated at different time of intervals for all the adsorbents used. The results are tabulated. It was found that the initial fluoride concentration drastically reduced and some are within and nearer to the permissible limits.
The schematic diagram and the experimental setup of column experiment are shown in Figures 6 and 7 respectively. A glass tube of inner diameter 3.5cm and length 60 cm was filled with the adsorbent as shown. The bottom of the tube was covered with a nylon cloth for ease flow; 50ml of water having a fluoride concentration of 5mg/l was poured into the column. The water was allowed to pass through the adsorbent at different flow rates with respect to time and was noted the passed water was collected in a beaker, and concentration of fluoride (Cf) in the water was measured for different flow rates using SPADN method, along with pH value. The same procedure has been repeated for all adsorbents and results were tabulated.
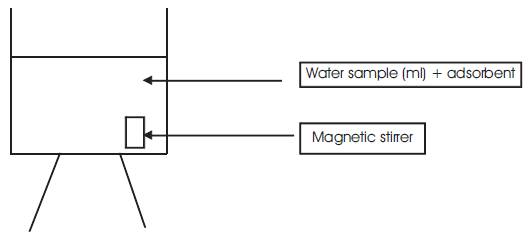
Figure 5. Experimental set up for Batch experiment
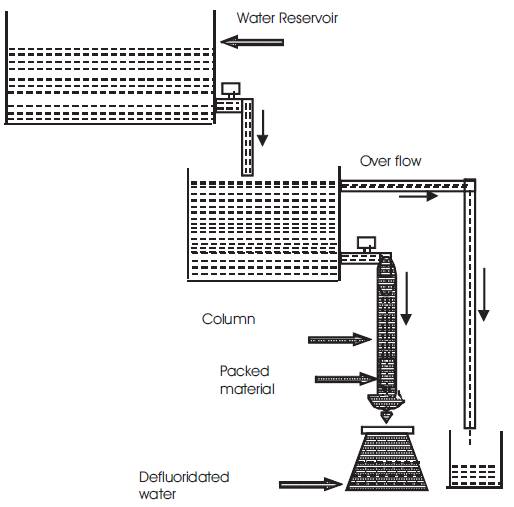
Figure 6. Schematic diagram of Column experiment

Figure 7. Experimental set up of column experiment
Table 1 and Table 2 give the details of the batch experimental test results and column experimental test results respectively for different adsorbents.
The above results can be depicted in the form of graph as shown in Figures 8 to 10.
Figures 8, 9 and 10 give the variation of fluoride concentration (Cf) in water with time (t) when different adsorbent used were neem leaves, amla powder, tamarind powder and brick powder respectively in batch experiment. Volume of water = 50ml, Cf = 2.5mg/l, particle size = 1.4- 1.7mm, mass of adsorbent added was 0.5g, 1.0g and 1.5g.
When neem leaves was used as an adsorbent to carry out batch experiment, it was found that fluoride concentration increased from 2.5mg/l to 3.8mg/l. Hence, this material is not suitable for defluoridation.
Figure 8 shows that at higher dosage of brick powder as adsorbent, concentration of fluoride (Cf) in water was decreased drastically upto first 600secs and thereafter started attaining a constant value of 1.9mg/l upto 2800secs gradually. But it imparts reddish brown color during defluoridation process. Here collected bricks are crushed into powder form and sieve analysis was done to obtain particle size of 1.4- 1.8mm.
Figure 9 shows that at higher dosage of tamarind powder as adsorbent, concentration of fluoride (Cf) in water were decreased rapidly upto first 450 secs and there after started attaining a constant value of 1.6mg/l upto 2900 secs gradually. But it imparts brownish tinge color and turbidity to the water after defluoridation process. Hence tamarind powder can also be used as one of the adsorbents for defluoridation of drinking water, but it can be improved further to remove the color.
Figure 10 shows that at higher dosage of amla powder as adsorbent, concentration of fluoride (Cf) in water decreased very rapidly upto first 500 secs and there after started attaining a constant value of 1.4mg/l upto 3000 secs gradually. But it imparts greenish yellow color along with little turbidity to the water after defluoridation process, and it can be improved further to remove the color. Hence amla can be used as adsorbent for defluoridation of drinking water.
The above results can be depicted in the form of graph as shown in Figures 11 to 14.
Figures 11, 12, 13 and 14 give the percentage reduction in fluoride concentration (Cf) in water with respect to flow rate lit/day in column experiment, when different adsorbent used were amla powder, tamarind powder and brick powder respectively and adsorbent added was 0.5g, 1.0g, 1.5g.
Figure 11 shows the effect of amla powder when added in different dosage like 0.5g, 1.0g and 1.5g to the column of diameter 3.5cm and length 60cm. The height of the packed bed media was 10cm. Fluorine contained water having a concentration of 5mg/l was allowed to pass through media and contact time was noted. Number of trials was done for different flow rates. It has been observed that amla powder reduced fluoride content in drinking water at the highest rate.
Figure 12 shows the effect of brick powder of grain size 1.2 mm when added and column was filled upto 10cm height. The height of the packed bed media was 10cm. Fluorine contained water having a concentration of 5mg/l was allowed to pass through media and contact time was noted. Number of trials was done for different flow rates, it has been observed that brick powder reduced fluoride content in drinking water upto 62%.
Figure 13 shows the effect of clay with 10% of sand when added and column is filled upto 10cm height. The height of the packed bed media was 10cm. Fluorine contained water having a concentration of 5mg/l was allowed to pass through media and contact time was noted. Number of trials was done for different flow rates, and it is has been observed that clay with 10% of sand as flow media reduced fluoride content in drinking water upto 58%.
Figure 14 shows the effect of clay with 20% of sand when added and column is filled upto 10cm height. The height of the packed bed media was 10cm. Fluorine contained water having a concentration of 5mg/l was allowed to pass through media and contact time was noted. Number of trials was done for different flow rates, and it is has been observed that clay with 20% of sand as flow media reduced fluoride content in drinking water upto 60%.
As the addition particle size increases from 0.5g to 1.5g, the removal capacity fluoride was increased. Tamarind powder was found to be less effective for defluoridation. Neem powder, when used as an adsorbent in fact increased the concentration of fluoride and hence tamarind powder is not suitable for this process.
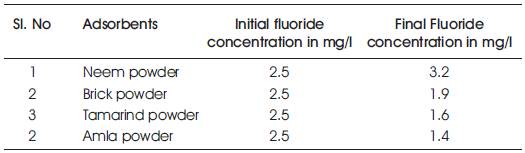
Table 1. Test results of fluoride concentration for different adsorbent.
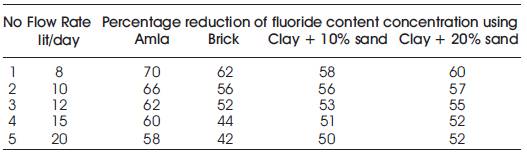
Table 2. Test results of fluoride concentration for different adsorbents and clay with different percentage of sand at different flow rate
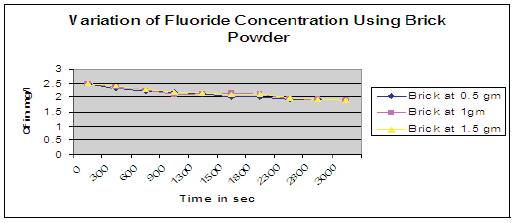
Figure 8. Variation of fluoride concentration using Brick powder
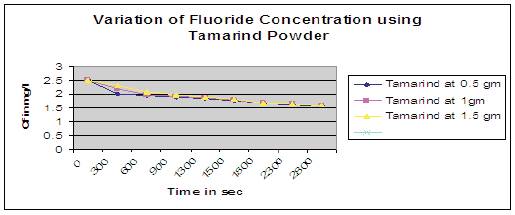
Figure 9. Variation of fluoride concentration using Tamarind powder
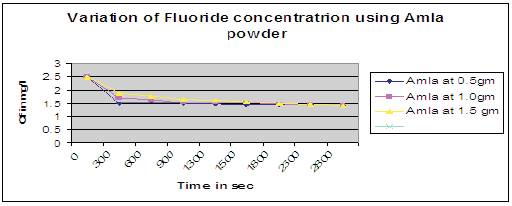
Figure 10. Variation of fluoride concentration using Amla powder
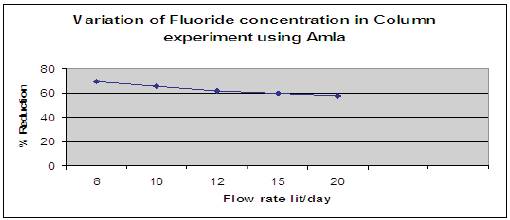
Figure 11. Variation of fluoride concentration using Amla powder.
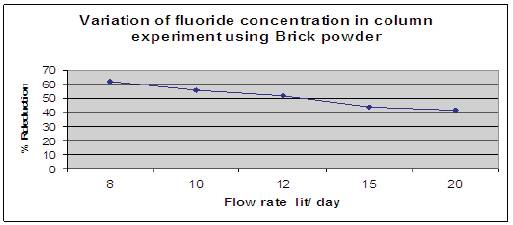
Figure 12. Variation of fluoride concentration using Brick powder.
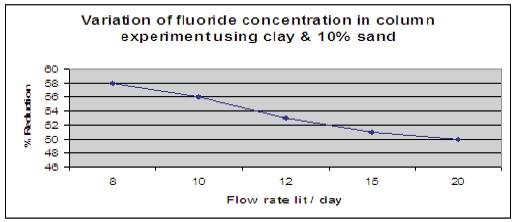
Figure 13. Variation of fluoride concentration using Clay with 10% Sand.
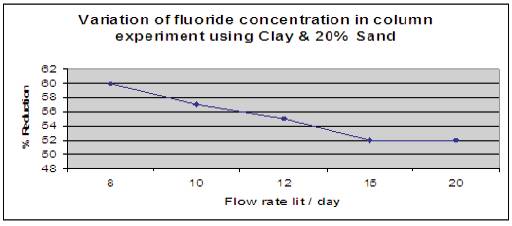
Figure 14. Variation of fluoride concentration using Clay with 20% Sand.
Based on experimental results obtained, the following conclusions can be drawn.
The authors would like to thank Vice-Chancellor, Christ University, Fr. Benny Thomas, Director and Dr. Iven Jose, Associate Dean, Christ University Faculty of Engineering, Bangalore for their constant encouragement.