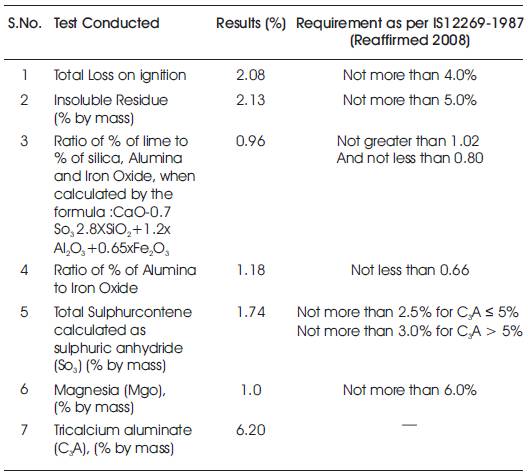
Table 1. Chemical Properties of Portland Cement (53 grade)
This paper presents durability of high volumes of Slag Concrete, exposed to Hydrochloric acid, Sulphuric acid and Sodium Sulphate. In the present investigation, high volumes of Slag Concrete of 100mm cubes of eight mixes were cast and cured for 28 days. The cubes were immersed in distilled water with 10% of Hydrochloric acid, 10% of Sulphuric acid and 10% of Sodium Sulphate separately. The durability studies of High Volumes of Slag Concrete were conducted at 28 days, 90 days, 180 days and 360 days. The results obtained are presented and compared between different mixes.
Concrete structures are built in highly polluted urban and industrial areas, aggressive marine environments, harmful sub-soil water in coastal areas and many hostile conditions where other materials of construction are found to be non-durable. Since the use of concrete in recent years have spread to highly harsh and hostile conditions, the earlier impression that concrete is a very durable material is being threatened particularly on amount of premature failures of number of structures in the recent past. Concrete is not fully resistant to acid. Most acid solutions will slowly or rapidly disintegrate Portland cement concrete depending upon the type and concentration of acid (M.S.Shetty, 2004, p.349 & 395).
Sulphates are commonly present in soil, ground water and sea water and effluent discharges from industry. Damage caused by sulphates has been observed particularly in foundations, sewage systems and marine structures. The traditional explanation about sulphate attack is: Sulfates react with calcium hydroxide (Ca(OH)2 ) forming gypsum. The gypsum may then react with tricalcium aluminate (C3A), in the concrete to form ettringite and monosulfoaluminate. These reactions cause substantial increase in volume with subsequent cracking and peeling (Elkem Materials, Sulfate Resistance, 2001, para. 2,3).
Shoichi Ogawa et al.'s 2008 study found the following: Asian GGBS (Ground Granulated Blast-furace Slag) contains an alumina content of around 15 mass% and this is considered too high for good sulfate resistance. The use of low- C3A cement is effective in improving sulphate resistance by suppressing the formation of ettringite, in severe sulphate environments. A novel GGBS showed excellent sulphate resistance although the simple GGBS blended slag cement expanded after half year in 5% Na2SO4 solution. The improving mechanism of the novel GGBS is the suppression of monosulfoaluminate formation while keeping aluminate hydrates in the form of Ett, as well as the reduction of CH that is another essential material for delayed Ett. formation and these reactions result in the suppressing of the formation of harmful Ett.(p. 500).
The main reason for the increased acid resistance of the concrete investigated was the formation of a very dense hardened cement paste and aggregate interface with very low porosities. The use of slag cement increased acid resistance, as well as Portland cements with silica fume (up to eight percent by mass of cementitious material) or fly ash(Acid Resistance, 2014, para.4). The addition of ultrafine pozzolan, such as silica fume, to a mixture containing blast-furnace slag can prevent excessive bleeding problems (Roland Bleszinski et al. 2002, p.499).
The overall durability of the Portland Slag Cement has been more than satisfactory even in the coastal constructions, as seen from comparatively low repair and rehabilitation efforts (A.K.Chattergee, 2000, p.387).
Elena Dan et al.'s (2003) study found the following: The resistance of SAB (Sulphoaluminate-Belite Cement) cement to 0.5 % HCl solution is even lower than that of low - resistant OPC (Ordianary Portland Cement) and Blast Furnace Slag Portland Cement (BFSPC). The primary reason of cements' low resistance is their high alkalinity. The resistance of SAB cement to sulphate attack when exposed to 5 % Na2SO4 solution is higher than that of sulphate non-resistant OPC and equivalent to the well - known sulphate resistant BFSPC. It seems that ettringite formation as an integral element of hydration process at the ambient conditions, and contemporarily the absence of C3A in SAB cement contribute in avoiding structural deterioration caused by extreme development of voluminous reaction sulphate - based products (pp.147-148).G.J. Osborne(1999) concluded that Structural concretes containing up to 50% of slag as cementitious material are considered suitable for the same uses, in ordinary and mild exposure applications, as conventional Portland cement concretes of the same design strength. Rates of carbonation and permeability to gaseous species are likely to be similar (p.20).
Wikipedia on GGBS (Ground Granulated Blast-furnace Slag) cement is frequently specified in concrete to provide protection against both sulphate attack and chloride attack. Most projects in Dublin's Docklands, are using GGBS in subsurface concrete for sulfate resistance. GGBS reduces thermal gradients in the concrete which prevents the occurrence of microcracking which can weaken the concrete and reduce its durability, and was used for this purpose in the construction of the Jack Lynch Tunnel in Cork (Architectural and Engineering Benifits, 2014, para.1).
The compound composition of many Portland cements shows that there has been a significant increase in C3S/C2S ratios of modern Portland cements from about 1.5 to 5.0. So, the change in chemical composition results in high early strength with lower rate of increment, further it causes high heat of hydration which results in “thermal cracking” (Swamy R.N, 2007, p.9).
The sulphuric acid attack causes degradation in the compressive strength of Geo-polymer concrete and the extent of degradation depends on the concentration of the acid solution and the period of exposure. However, the sulfuric acid resistance of Geo-polymer concrete is significantly better than that of ordinary Portland cement concrete (Neetu Singh et al., 2013, p.283). William Aperador Chaparro et al. (2011) concluded the following: For 0 months immersion (28 days of curing), the AAS (Alkali-Activated Slag) and OPC concretes presented a 10% probability of corrosion. After 3 months of immersion, the tested AAS and OPC concretes showed similar behavior. The active potentials in the range from 0.2 to 0.6 V vs. Cu/CuSO4, indicate a 90% probability of corrosion.
Elke Gruyaert et al.'s (2012) study revealed the following: BFS concrete, exposed to a lactic–acetic acid solution (pH~2), performs significantly better than OPC concrete. The concrete's acid resistance does not improve considerably if the cement replacement percentage is increased from 50% to 85% (remember however that the compressive strength of S50 equals that of S0, while S85 has a considerably lower mechanical performance). Since lactic and acetic acids leach out Ca-ions, the better performance of BFS concrete was mainly due to the lower CaO content and the higher SiO2content of BFS. Because of the higher CH content and higher C/S ratio in the cement hydration products, OPC concrete is more vulnerable towards acid attack: the attacked layer is porous, has almost no mechanical strength and can be easily removed by brushing. In BFS concrete, the CH content is strongly reduced, the C/S ratio in the hydration products is lower and decalcification leads to the formation of a silica(alumino)gel. From literature review, it seemed that this layer hinders the further ingress of acids and contributes towards the better acid resistance of BFS concrete(p.183).
Donald W. Lewis March(1981) stated that Sulfate resistance is affected by the alumina content of the slags, with high alumina slag (about 17%) reported to decrease sulfate resistance at low percentages of cement replacement (50% or less). Low alumina slags (11%) seem to increase sulfate resistance at all levels of use. With high replacement levels (65% or more), all slags increase the sulfate resistance compared to the Portland cement used with them (p.1). David N. Richardson (2006) stated, Freeze-thaw durability was lower for the slag- PC field mixes than the plain PC field mix. However, under optimum wet plus dry curing periods, the slag-PC mix Durability Factors approached that of the plain OPC mix: (p.74). Roa-Rodriguez.G's (2014) referred the following: Referring to concrete made with Portland cement, certain mineral acids such as HCl, HNO3 and H2SO4 can be very aggressive because of the alkaline nature of the reaction products, leading to the complete destruction of the material (p.282).
Ground granulated blastfurnace slag (GGBFS) cement is a by-product of the manufacture of iron in which limestone or dolomite is used as flux material. OPC and GGBFS have similar chemical composition. But the minor differences between GGBS and OPC also give enhanced durability to concrete made with GGBS.In the present study, an attempt has been made to assess the suitability of Ground granulated blast furnace slag as cement replacement material in concrete making. In this study, High Volumes of Slag Concrete of 100mm cubes of eight mixes of w/binder ratios from 0.27 to 0.55 were casted and cured for 28 days. The cubes were immersed in distilled water with 10% of Hydrochloric acid (HCl), 10% of Sulphuric acid (H2SO4) and 10% of Sodium Sulphate (Na2SO4) separately. The durability studies of High Volumes of Slag Concrete are conducted in terms of weight loss and strength loss at 28 days, 90 days, 180 days and 360 days and also comparison is made between them.
Locally available 53 grade of Ordinary Portland Cement confirming to IS: 12269 was used in the investigations. The cement is tested for various properties like Normal consistency, specific gravity, Fineness, Soundness, Compressive Strength and Specific Surface area, and were found to be 28%, 3.10, 4%, 0.5 mm, 53Mpa and 3100 cm2/g respectively in accordance with IS:12269-1987. The chemical properties of Ordinary Portland Cement are shown in Table.1.
GGBS which is available locally was procured from Steel Plant, Visakhapatnam (Dt.), Andhra Pradesh. The physical requirements in accordance with IS 1727- 1967 (Reaffirmed 2008) and chemical requirements in accordance with IS: 12089 – 1987 (Reaffirmed 2008) are shown in Tables 2 & 3.

Table 1. Chemical Properties of Portland Cement (53 grade)
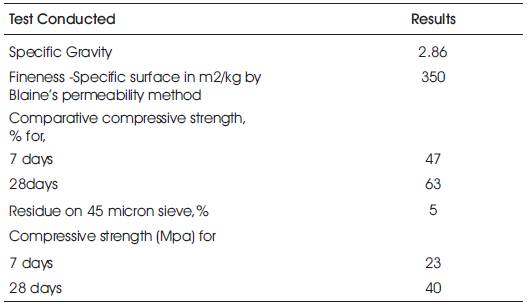
Table 2. Physical Properties of GGBFS – Technical Ref.: S 1727-1967(Reaffirmed2008)
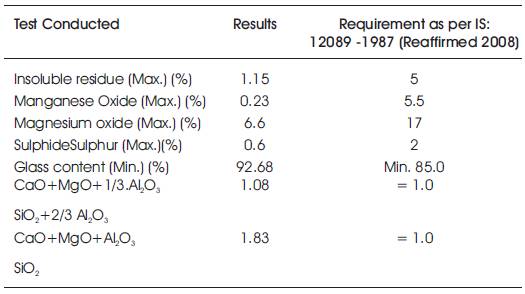
Table 3. Chemical Composition of GGBFS: As per IS: 12089 – 1987 (Reaffirmed 2008)
The Super plasticizer utilized was supplied by internationally reputed admixture manufacturers. Endure flowcon04 was manufactured by Johnson. Endure flowcon04 is dark brown colored liquid and it is based as sulphonatednaphthalene formaldehyde (SNF) super plasticizer. It complies with IS: 9103-1999, Bs5075 and, ASTM C-494, was used. The super plasticizer is tested for properties like density and pH which were found to be 1.2 and minimum 6.
The locally available river sand is used as fine aggregate in the present investigation. The sand is free from clay, silt and organic impurities. The sand is tested for various properties like specific gravity, water absorption and fineness modulus of fine aggregate were found to be 2.55,1.72 and 2.74 respectively in accordance with IS:2386- 1963.
Machine crushed angular granite metal of 20mm nominal size from the local source is used as coarse aggregate. It is free from impurities such as dust, clay particles and organic matter etc. The coarse aggregate is also tested for its various properties. The specific gravity, water absorption, bulk density and fineness modulus of coarse aggregate were found to be 2.60, 0.38, 1490 kg/m3 and 7.16 respectively.
Locally available water was used for mixing and curing which is potable, clean and free from injurious amounts of oils, acids, alkalis, salts, sugar, organic materials or other substances that may be deleterious to concrete or steel.
After 28 days of curing, each cube is tested for weight and compressive strengths. The cubes are subjected to 10% solutions of Sulphuric Acid (H2SO4), Sodium Sulphate (Na2SO4) and Hydrochloric acids (HCl) individually. Cubes are continuously immersed in solution for 28days, 90days, 180days, and 360days. The specimens are arranged in the plastic baths in such a way that the clearance around and above the specimen is not less than 30mm.The response of the specimens to the solution is evaluated through change in weight and compressive strength. Three specimens from each group are used for testing after 28days, 90days, 180days and 360days of immersion. Before testing, each specimen is removed from the baths, brushed with a soft nylon brush and rinsed in tap water. This process removes loose surface material from the specimens. For determining the resistance of concrete specimens to aggressive environment such as acid attack, the durability factors are considered as proposed by the philosophy of ASTM (666-1997). In the present investigation, the “Acid Durability Factors” are derived directly in terms of relative strengths. The relative strengths are always compared with respect to the 28 days value (i.e. at the start of the test). The “Acid Durability Factors” (ADF) can be calculated as follows.
| ADF = Sr N / M | (1) |
Where, Sr - Relative Strength at N days, (%)
N - Number of days at which the durability factor is needed.
M - Number of days at which the exposure is to be terminated.
So M is 360 in this case.
The quantities of materials for one cubic meter of high volumes of slag concrete and also the data obtained from the tests on cement, GGBS, aggregates, admixture and water are shown in Table 4.
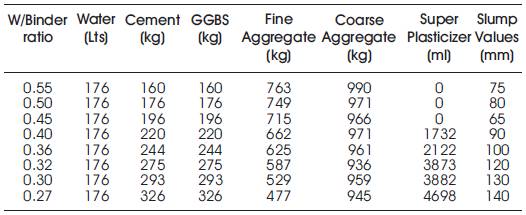
Table 4. Quantities of Material required per One Cu. m. of High Volumes of Slag Concrete
The workability of different concrete mixes was measured using slump test. It can be seen from Table 4 that a medium workability was maintained for almost all the mixes by addition of suitable quantities of super plasticizer.
High volume slag concrete strength decreased as the different water/binder ratios increased from 0.27 to 0.55.The hydrated product of cement compound in a grain of cement adheres firmly to the unhydrated core in the grains of cement. That is to say unhydrated cement left in grain of cement will not reduce the strength of cement mortar or concrete as long as the products of hydration compact. This contributes to increase in strength of high volumes of slag concrete of lower water binder ratios. In addition to strength, durability property is also important for concrete. When concrete is exposed to aggressive environment consisting of chloride and sulphates, it is subjected to deterioration. Pozzalonic admixtures such as GGBFS, etc., when used in concrete are known to enhance the durability property of concrete.
To study the effect of GGBFS as on addition material to concrete, acid and sulphate resistance test have been conducted in the laboratory specimens of concrete cured for 28 days and exposed to 10% HCL, 10% H2SO4and 10% NA2SO4 as shown in Figure 1. The Compressive strengths of Cubes before exposure to acid and sulphate are shown in Table 5. The period of exposure is kept as 28, 90, 180& 360 days. The specimens of W/b ratio 0.55, 0.50, 0.45, 0.40, 0.36, 0.3 and 0.27 of High Volumes of Slag Concrete have been tested for durability. The results of acid and sulphate resistance of tests conducted on the above specimens are given in Tables 6 to Table 13.
From Table 6 & Figure 2 it can be seen that the percentage loss in weight for w/b ratios varying from 0.27 to 0.55 are observed from 0.89% to 1.75% at 28days, 2.76% to 5.03% at 90 days, 5.33% to 6.9% at 180 days and 7.1% to 9.8% at 360 days of exposures respectively. For HVSC with lower w/b ratio, the percentage loss in weight is less during all days of exposure of 28, 90, 180 and 360 days. For higher w/b ratio the percentage loss in weight is higher for longer periods of exposure, whereas under the same period consideration the percentage loss in weight is less for lower w/b ratios. From Tables 5 & 6 and Figures 3 & 4, it can be seen that the percentage loss in strength for w/b ratios varying from 0.27 to 0.55 are observed from 0.84% to 1.91 % at 28days, 5.28% to 6.42% at 90 days, 6.30% to 8.39% at 180 days and 6.69% to 14.2% in 360 days of exposures respectively. For lower w/b ratio, the percentage loss in strength is less during all days of exposure of 28, 90, 180& 360 days. For higher w/b ratio, the percentage loss in strength is higher for longer periods of exposure, whereas under the same period consideration the % loss of strength is less for lower w/b ratios. Figures 5 & 6 show the immersion of cubes in HCl and after immersion in HCl. Table 7 shows relative strengths and acid durability factors of HVSC. Relative strengths and acid durability factors increases with decrease in water binder ratios.
From Table 9 & Figure 7, it can be seen that the percentage loss in weight for w/b ratios varying from 0.27 to 0.55 are observed from 5.38% to 10.41% at 28days, 7.67% to 14.72% at 90 days, 7.88% to 15.46% at 180 days and 9.78% to 17.32% at 360 days of exposures respectively. For lower w/b ratio, the percentage loss in weight is less during all days of exposure of 28, 90, 180 and 360 days. For higher w/b ratio, the percentage loss in weight is higher for longer periods of exposure, whereas under the same period consideration the percentage loss in weight is less for lower w/b ratios.
From Tables 8 & 9, Figures 8 & 9, it can be seen that the percentage loss in strength for w/b ratios varying from 0.27 to 0.55 are observed from 48.9% to 53.59% at 28days, 51.42% to 55.4% at 90 days, 54.43% to 58.58% at 180 days and 55.25 to 62.64% at 360 days of exposures respectively. For lower w/b ratio, the percentage loss in strength is less during all days of exposure of 28, 90, 180 and 360 days. For higher w/b ratio, the percentage loss in strength is higher for longer periods of exposure, whereas under the same period consideration the % loss of strength is less for lower w/b ratios. Figure 10 shows the after immersion in H2SO4. Table 10 shows relative strengths and acid durability factors of HVSC. Relative strengths and acid durability factors increases with decrease in water binder ratios.
From Table12 & Figure 11, it can be seen that the percentage loss in weight for w/b ratios varying from 0.27 to 0.55 are observed from 0.83% to 1.20 % at 28days, 1.03% to 1.5% at 90 days, 1.24% to 1.9% at 180 days and 1.32% to 7.86% at 360 days of exposures respectively. For lower w/b ratio, the percentage loss in weight is less during all days of exposure of 28, 90, 180 and 360 days. For higher w/b ratio, the percentage loss in weight is higher for longer periods of exposure, whereas under the same period consideration the % loss in weight is less for lower w/b ratios. Table 11 shows Compressive Strengths of High volumes of slag concrete specimens immersed in 10% of Na2SO4at 28 Days, 90 Days, 180 Days and 360 Days.
From Tables 12 & 13, Figures 12 & 13, it can be seen that the percentage loss in strength for w/b ratios varying from 0.27 to 0.55 are observed from 0.46% to 1.51 % at 28days, 1.3% to 1.89% at 90 days, 1.48% to 2.37% at 180 days and 2.01% to 4.03% 360 days of exposures respectively. For lower w/b ratio, the percentage loss in strength is less during all days of exposure of 28, 90,180 and 360 days. For higher w/b ratio, the percentage loss in strength is higher for longer periods of exposure, whereas under the same period consideration the % loss of strength is less for lower w/b ratios. Hence high volumes of slag concrete for lower water/binder ratios as contributed to a more durable concrete. Figures 14 & 15 show the immersion of cubes in Na2SO4and after immersion in Na2SO4. Table 13 shows relative strengths and acid durability factors of HVSC. Relative strengths and acid durability factors increases with decrease in water binder ratios. Figure 16 shows testing of specimen for compressive strength.
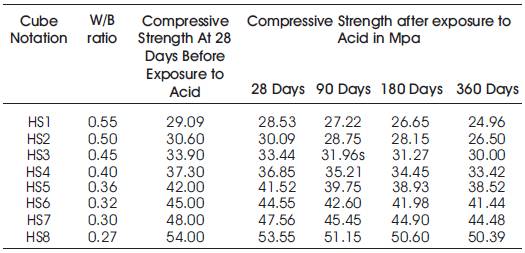
Table 5. Compressive Strengths of High volumes of slag concrete specimens immersed in 10% of HCl at 28 Days, 90 Days, 180 Days and 360 Days.
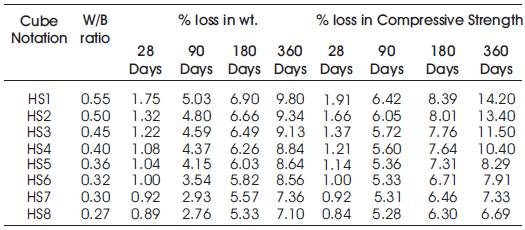
Table 6. Percentage weight loss and Percentage loss in Compressive Strength of specimens of High Volumes of Slag Concrete immersed in 10% HCL
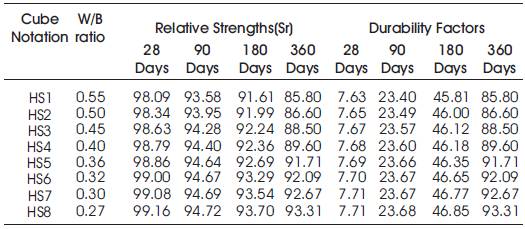
Table 7. Acid Durability factors (ADF) of High volumes of slag concrete specimens immersed in 10% of HCl at 28 Days, 90 Days, 180 Days and 360 Days

Figure 1. Immersion of cubes
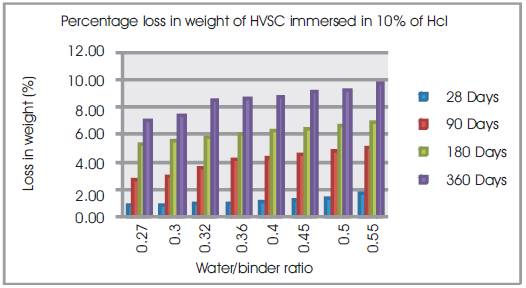
Figure 2. Percentage loss in weight of HVSC immersed in 10% of HCl
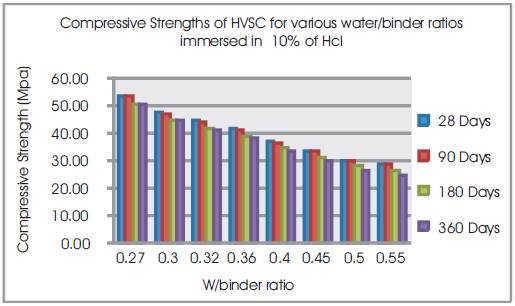
Figure 3. Compressive Strengths of HVSC for various water/binder ratios immersed in 10% of HCl
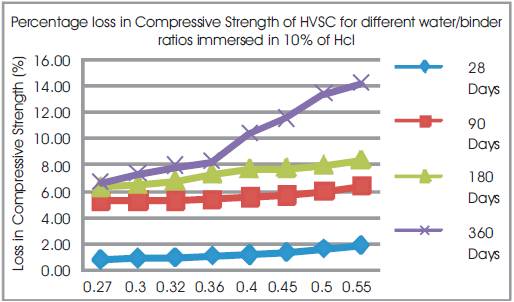
Figure 4. Percentage Loss in Compressive Strengths of HVSC for different water/binder ratios immersed in 10% of HCL

Figure 5. Immersion of cubes in HCl acid

Figure 6. HVSC after immersion in HCL at 360 Days
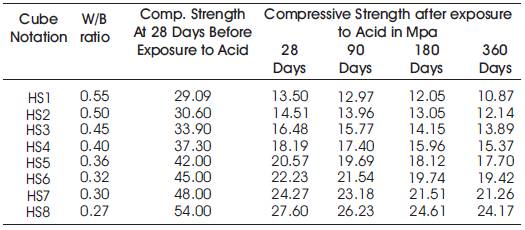
Table 8. Compressive Strengths of High volumes of slag concrete specimens immersed in 10% of H2SO4at 28 Days, 90 Days, 180 Days and 360 Days
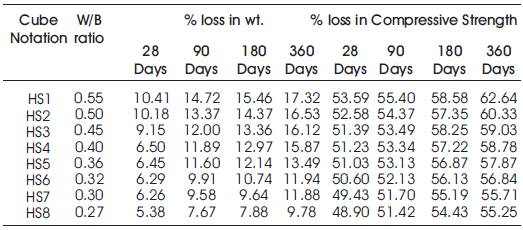
Table 9. Percentage weight loss and Percentage loss in Compressive Strength of specimens of High Volumes of Slag Concrete immersed in 10% H2SO4
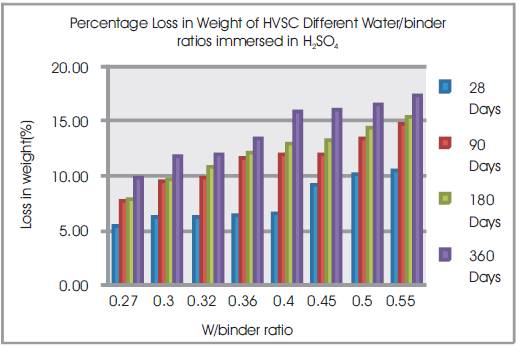
Figure 7. Percentage Loss in Weight of HVSC Different Water/binder ratios immersed in H2SO4
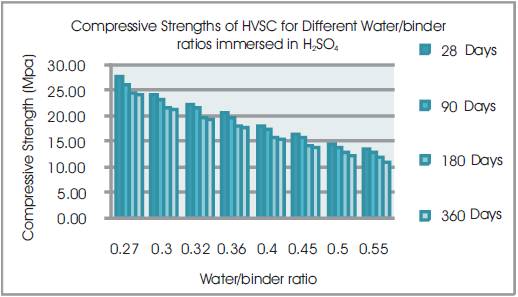
Figure 8. Compressive Strengths of HVSC for Different Water/binder ratios immersed in H2SO4
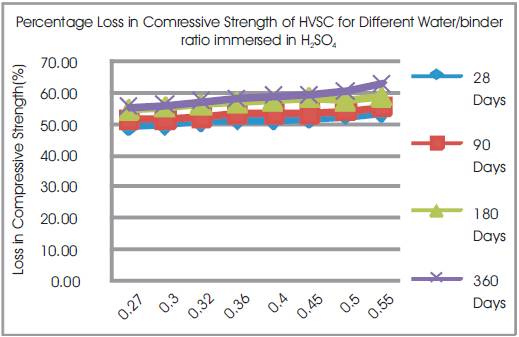
Figure 9. Percentage Loss in Comressive Strength of HVSC for Different Water/binder ratio immersed in H2SO4

Figure 10. HVSC after immersion in H2SO4 at 360 Days
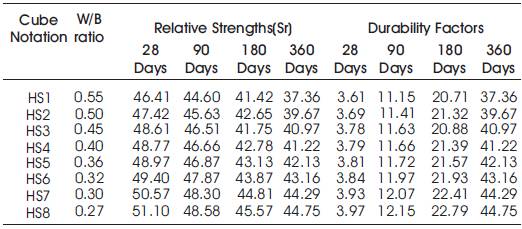
Table 10. Acid Durability factors (ADF) of High volumes of slag concrete specimens immersed in 10% of H2SO4 at 28 Days, 90 Days, 180 Days and 360 Days
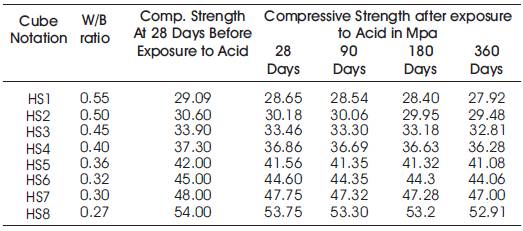
Table 11. Compressive Strengths of High volumes of slag concrete specimens immersed in 10% of Na2SO4at 28 Days, 90 Days, 180 Days and 360 Days
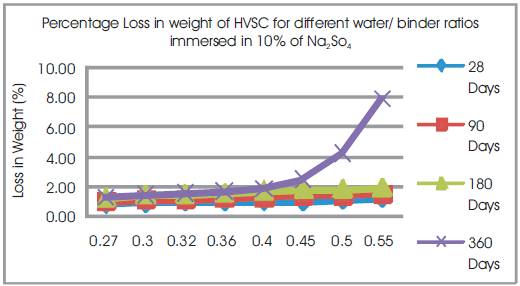
Figure 11. Percentage Loss in weight of HVSC for different water/ binder ratios immersed in 10% of Na2So4
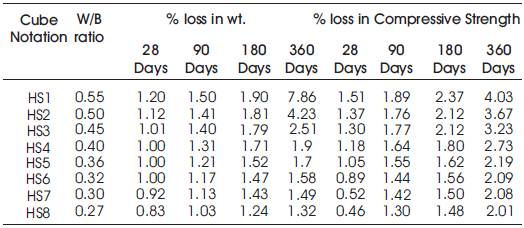
Table 12. Percentage weight loss and Percentage loss in Compressive Strength of specimens of High Volumes of Slag Concrete immersed in 10% Na2SO4
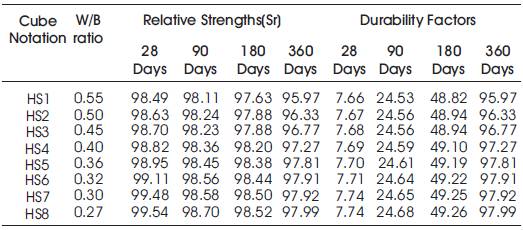
Table 13. Acid Durability factors (ADF) of High volumes of slag concrete specimens immersed in 10% of Na2SO4 at 28 Days, 90 Days, 180 Days and 360 Days
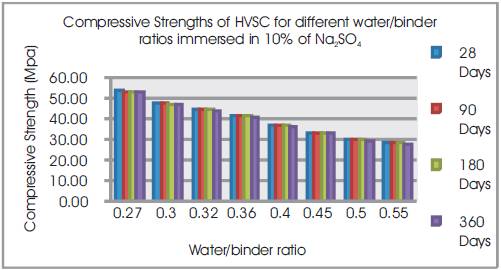
Figure 12. Compressive Strengths of HVSC for different water/binder ratios immersed in 10% of Na2SO4
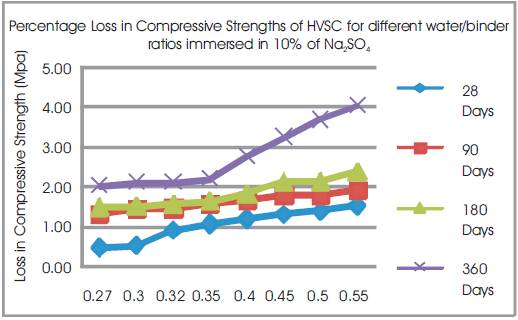
Figure 13. Percentage Loss in Compressive Strengths of HVSC for different water/binder ratios immersed in 10% of Na2SO4

Figure 14. Immersion of cubes in Na2SO4 acid

Figure 15. HVSC after immertsion Na2SO4 at 360 Days

Figure 16. Testing of Specimen
The following conclusions are drawn from experimental results: