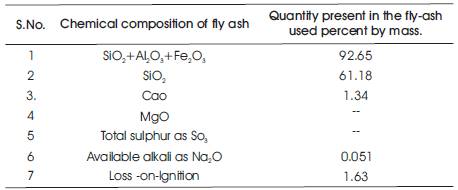
Table 1. Chemical composition of fly ash used
This paper presents the effect of Alkali activator / Fly ash ratio (A/F) on strength and workability of geopolymer concrete prepared from low lime based fly ash and a mixed alkali activator of sodium hydroxide and sodium silicate solution. The workability of concrete is found to be increasing when the A/F ratio is increased. An increase in compressive strength of the concrete samples is observed when the A/F ratio is increased for the samples cured at 60oc.
Concrete usage around the world is second only to water. Ordinary Portland cement (OPC) is conventionally used as the primary binder to produce concrete. The environmental issues associated with the production of OPC are well known. The amount of the carbon dioxide released during the manufacture of OPC due to the calcination of limestone and combustion of fossil fuel is in the order of one ton for every ton of OPC produced. In addition, the extent of energy required to produce OPC is only next to steel and aluminium. On the other hand, the abundant availability of fly ash worldwide creates an opportunity to utilize this by-product of burning coal, as a substitute for OPC to manufacture concrete. When used as a partial replacement of OPC, in the presence of water and in ambient temperature, fly ash reacts with the calcium hydroxide during the hydration process of OPC to form the calcium silicate hydrate (C-S-H) gel. The development and application of high volume fly ash concrete, which enabled the replacement of OPC up to 60% by mass (Malhotra 2002; Malhotra and Mehta 2002), is a significant development.
In 2001, the annual production of fly ash in the USA was about 68 million tons. Only 32 percent of this was used in various applications, such as in concrete, structural fills, waste stabilization/solidification etc. In 2000 ash production in Australia was approximated as 12 million tons, of which only about 5.5 million tons have been utilized (Heidrich 2002). Worldwide, the estimated annual production of coal ash in 1998 was more than 390 million tons. The main contributors for this amount are China and India. Only about 14 percent of this fly ash is utilized, while the rest was disposed in landfills (Malhotra 1999). By the year 2012, the amount of fly ash produced worldwide is estimated to be about 780 million tons annually (Malhotra 2002). The utilization of fly ash, especially in concrete production, has significant environmental benefits, viz, improved concrete durability, reduced use of energy, diminished greenhouse gas production, reduced amount of fly ash that has to be disposed in landfills, and saving of the other natural resources and materials.
In 1978, Davidovits proposed that an alkaline liquid could be used to react with the silicon (Si) and the aluminium (Al) in a source material of geological origin or in by product materials such as fly ash and rice husk ash to produce binders. Because the chemical reaction that takes place in this case is a polymerization process, (Davidovits 1994, 1999) coined the term 'Geopolymer' to represent these binders. Geopolymers are members of the family of inorganic polymers. The chemical composition of the geopolymer material is similar to natural zeolitic materials, but the microstructure is amorphous instead of crystalline (Palomo et al. 1999; Xu and van Deventer 2000). The polymerization process involves a substantially fast chemical reaction under alkaline condition on Si-Al minerals, that results in a three dimensional polymeric chain and ring structure consisting of Si-O-Al-O bonds, as follows (Davidovits 1999):
Mn [-(SiO2) z–AlO2] n . wH2O
Where: M is the alkaline element or cation such as potassium, sodium or calcium;
The symbol – indicates the presence of a bond,
n is the degree of polycondensation or polymerization
z is1,2,3, or higher, up to 32.
The strength of geopolymer depends on the nature of source materials, Geopolymers made from calcined source materials, such as metakaolin (calcined kaolin), fly-ash, slag etc., yield higher compressive strength when compared to those synthesized from non-calcined materials, such as kaolin clay. A combination of sodium or potassium silicate and sodium or potassium hydroxide has been widely used as the alkaline activator. Because heat is a reaction accelerator, curing of a fresh geopolymer is carried out mostly at an elevated temperature.
Low-calcium fly ash is preferred as a source material than high calcium fly ash. The presence of calcium in high amount may interfere with the polymerization process and alter the microstructure (Gourley2003). In the present investigation work, the fly-ash used is obtained from Ramagundam Thermal Power Station (RTPS) in Andhra Pradesh. Chemical analysis of fly-ash is shown in Table 1 and the fly-ash conforms to the various specifications of IS : 3812-1987.

Table 1. Chemical composition of fly ash used
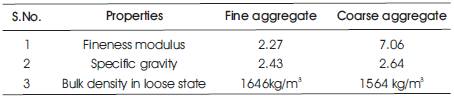
Table 2. Physical properties of aggregates used in the mix
The sodium hydroxide (NaOH) used in this study was in flake form and of 98% purity. The sodium silicate solution (Na2SiO3) used is available locally. The composition of the solution is Na2O=12.65%, SiO2=29.93%, H2O=56.42% by mass.
To improve the workability of the fresh geopolymer concrete Super plasticizer (CONPLAST SP-430) is used in the mix and the dosage of the Super plasticizer is fixed to 1.5% of the mass of fly-ash (Hardjito D & Rangan B.V, 2005).
Three types of locally available aggregates, viz. 6mm, 20mm coarse aggregates and fine sand in saturated surface dry condition were mixed together in the ratio 0.3:0.3:0.4 respectively.
To start with, the following parameters are fixed as shown in Table 3, based on estimations and results of previous experiments submitted in the research report by (Hardjito D & Rangan B.V, 2005).
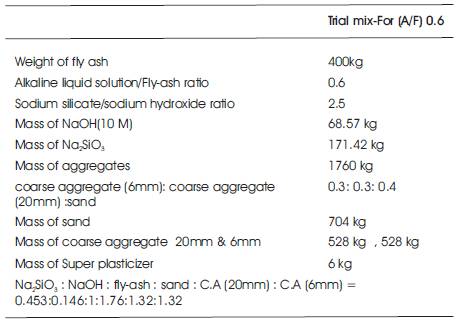
Table 3. Trial mix proportions for 1m3 of Geopolymer concrete for 10M NaOH solution
The Workability results of Slump for two mixes are presented in Table 4 and the results show that as the Molarity of NaOH solution is increased, the slump decreases and as A/F ratio is increased the slump increases for any concentration of NaOH solution (Figure 1).
The compressive strength results are presented in Tables 5 & 6 for the concrete mixes as an average of 6 readings .The compressive strength of concrete increases with the age for a particular molarity of NaOH solution and the 28 day compressive strength of concrete increases as the molarity of the NaOH solution is increased (Figures 2 & 3). As A/F ratio is increased the compressive strength is found to be increasing for all concentrations of NaOH solution (Figures 4 & 5).
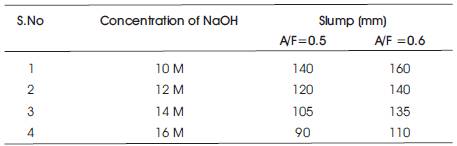
Table 4. Workability of Geoploymer concrete
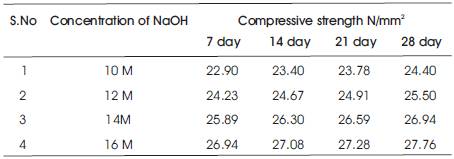
Table 5. Compressive strength of Geopolymer concrete with A/F 0.5 cured at 60oC
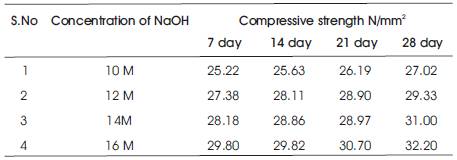
Table 6. Compressive strength of Geopolymer concrete with A/F 0.6 cured at 60oC
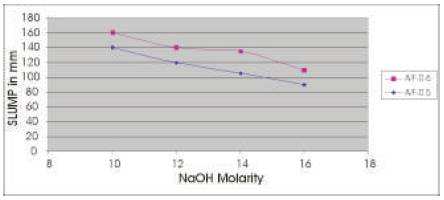
Figure1. Variation of Slump with Concentration of NaOH solution for different A/F ratio
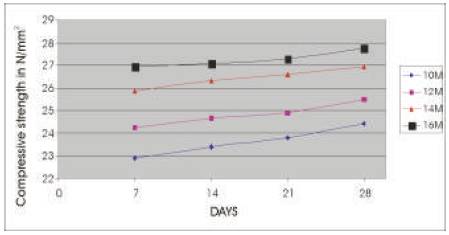
Figure 2. Relationship between Compressive strength of Geoploymer concrete and age for different Concentrations of NaOH solution (A/F 0.5)
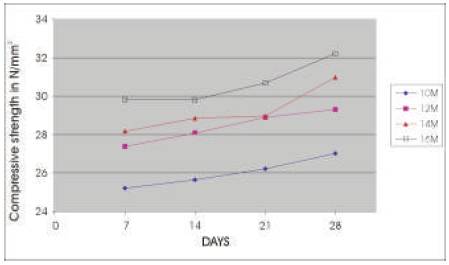
Figure 3. Relationship between Compressive strength of Geoploymer concrete and age for different Concentrations of NaOH solution (A/F 0.6)
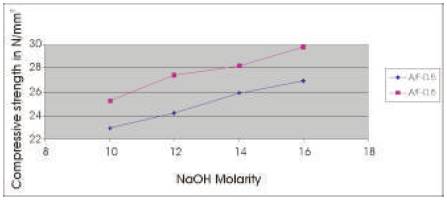
Figure 4. Comparison between 7 day Compressive strength of Geoploymer concrete for Concentrations of NaOH solution and A/F ratio
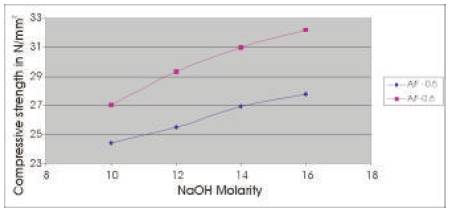
Figure 5. Comparison between 28 day Compressive strength of Geoploymer concrete for Concentrations of NaOH solution and A/F ratio

Figure 6. Fly ash being weighed for mixing

Figure 7. Mixing of geopolymer concrete using pan mixer

Figure 8. Casting of GPC in moulds

Figure 9. Compacting of concrete using vibrator
From the experimental results on the low lime fly ash based Geoploymer concrete, the conclusions are drawn as follows: