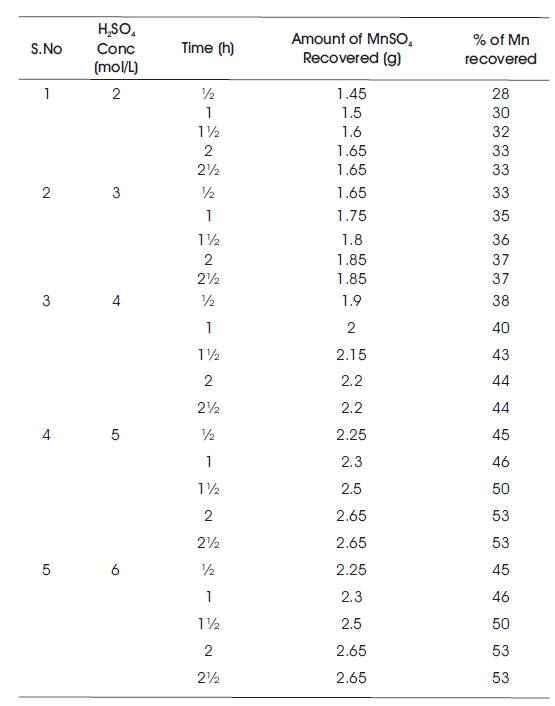
Table 1. Temperature at 40oC
The current study assesses the prospect of selective leaching of Manganese (Mn) from complex, in a high temperature sulphuric acid medium. The factors affecting the sulphuric acid leaching of the manganese complex in presence of various parameters were investigated. The three variables studied were temperature of leaching medium, leaching time, and concentration of sulphuric acid. Experiments were carried out at temperature ranging from 40o C to 110o C, time from 30 minutes to 150 minutes and sulphuric acid concentration from 2% to 6%. The feed ratio is 1:3 (5g MnSO4 + 15g impurities). The feed prepared is in close resemblance to pyrolusite ore. Manganese leaching amount was measured by using the standard manganese concentration curve and estimated by titration method. Effects of various acid concentrations on leaching efficiency were studied. The optimum conditions established for maximum metal extraction are: 100oC temperature, 120 minutes time, and 5% concentrated sulphuric acid. Under these conditions, recovery of Mn was 98%, and the leached residues were analyzed. The present process may find application of separation of manganese from iron and aluminium at high temperature during various hydrometallurgical treatment of manganese based ores.
Metals have been in service of mankind since the early days of civilization. The history of mankind from time immemorial shows intimate and in alienable relationship with minerals and metals. Mineral-metals play significant role for the economy and industrial growth of any developing nation. The birth of hydrometallurgy resulted from the discovery that the water dripping from the roof of underground mine working or flowing out of ore dumps often contain metallic compounds and that these compounds could be readily recovered by simply letting the liquor dry up in suitable pond. Metals in the minerals are generally present as carbonates, oxides, and sulphides. The minerals in which the metals are sufficiently concentrated and form commercial source of desired metals are called ore. A process applied to obtain pure metals after excavation of ore from the mine is referred to as Mineral Leaching. In the recovery of noble metals, including gold, silver and members of the platinum group, different leachants are used namely, sodium cyanide, thiourea, chlorine, sulphuric acid and aqua regia, and optionally a leaching aid, such as chloride ions. The most important manganese ore is pyrolusite (MnO ). Other 2 economically important manganese ores usually show a close spatial relation to the iron ores, where land-based resources are large but irregularly distributed.
There are several methods used to recover Manganese by Leaching; of which a few important techniques were reviewed from Literature.
A more progressive extraction process involves directly reducing manganese ore in a heap leach. This is done by percolating natural gas through the bottom of the heap; the natural gas provides the heat (needs to be at least 850oC), and the reducing agent (carbon monoxide). This reduces all of the manganese ore to manganese oxide (MnO2 ), which is a leachable form. The ore then travels through a grinding circuit to reduce the particle size of the ore to between 150-250 μm and this increases the surface area to aid in the leaching process(Abbruzzese, 1987; Cheng, Zhu, & Zhao, 2009). The ore is then added to a leach tank, which contains sulphuric acid and ferrous iron (Fe2+ ) in a 1.6:1 ratio. The iron reacts with the manganese dioxide to form iron hydroxide and elemental manganese. This process yields approximately 92% recovery of the manganese. For further purification, the manganese can then be sent to an electro-winning facility (Ghosh, Barik & Anand, 2008; Mishra, Srivastava, Sahu, Singh & Jana, 2011).
The ammonium sulphate process is described in U.S. Patents 1,889,021; 1,937,508; 1,947,457; 1,951,341; 2,074,013. Manganese is reduced to MnO and Fe2O3 is reduced to Fe3O4 by a reducing roast. The calcine, which must be kept away from air to prevent re-oxidation, is agitated with ammonium sulphate liquor at about 88 °C. MnO dissolves to form MnSO4 with the generation of ammonia, which is recovered. Manganese can be recovered from the sulphate liquor by the methods like crystallization, electrolysis, precipitation with lime (Mohammad, Saeid & Nooshin, 2012; Mohwinkel, Kleint & Koschinsky, 2014).
The ore, usually containing around 20 percent of Mn, is crushed and ground to minus 60-mesh and fed to a reducing kiln, where manganese is reduced to MnO and iron to Fe3O4 (insoluble in nitric acid). The reduced ore is leached with nitric acid in stainless steel equipment, sulphuric acid being added to precipitate barium, calcium and lead. The temperature in the leaching vessels rises to 80-90 °C and the leach is finished within an hour. After filtration, the insoluble residue is washed and the wash solution is used to dilute the nitric acid for the next leach(Randhawa, Hait & Jana, 2015; Samanta, Ojha, Madal & Sarkar, 2013). The Mn(NO3)2 solution, which may contain some Na, K, Mg, and Zn, is concentrated in an evaporator and decomposed in the presence of air at 200 °C. The decomposition takes place on the surface of an internally heated rotary drum inside the stainless steel decomposition unit. This drum dips into a pan filled with concentrated Mn(NO3)2 solution and is covered with a layer of the solution. The MnO2 is removed from the drum with a knife and discharged on a chute to the outside. Nitric acid leaves with the air which is sucked through the system, and is condensed to a liquid containing about 50 per cent HNO3 . The air is then washed with water to remove residual HNO3 . The MnO2 leaving the decomposition unit is washed to remove soluble impurities, dried and nodulised to produce a product containing 60 percent Mn(Su, Wen, Wang, Sun & Tong, Z., 2008; Tan, Li, Liu, Qu, Xia, Hu & Li, 2014).
Manganese is not found as a free element in nature; it is often found in combination with iron, and in many minerals. Manganese is a metal with important industrial metal alloy uses, particularly in stainless steels. The most important manganese ore is pyrolusite (MnO2 ). Other economically important manganese ores usually show a close spatial relation to the iron ores. Land-based resources are large, but irregularly distributed(Tang, Zhong, Wang, Li, & Liu, 2014). Attempts to find economically viable methods of harvesting manganese nodules were abandoned in the 1970s. Pure manganese used for the production of iron-free alloys is produced by leaching manganese ore with sulfuric acid and a subsequent electrowinning process(Tian, Wen, Tang, Liang, Pi, & Wang 2010). Leaching is the process by which constituents of a solid material are released into a contacting water phase. Although some species may be more of an environmental concern than others, the leaching process is in discriminant such that all constituents (e.g., major or minor matrix components as well as inorganic, organic and radionuclide contaminants) are released under a common set of chemical phenomena, which may include mineral dissolution, desorption and complexation, and mass transport processes. In turn, these phenomena are affected by certain factors that can alter the rate or extent of leaching (Xue, Zhong, Wang, Li, Li, & Wu, 2014; Zhuo, Yue, & Wen, 2009).
The factors are:
Considering all these factors, this study is carried out for recovery of Manganese by Leaching with Sulfuric acid (Barik, et al., 2015).
It consists of heating mantle, round bottom flask, rubber stoppers, thermometer, funnel, filter paper, and specific gravity bottle. The chemicals used are manganous sulphate, sulphuric acid, and sand.
The process variables considered are sulphuric acid concentration, temperature, and time as given as follows,
It starts with the preparation of the ore material of manganous sulphate and sand mixture in 1:1 ratio. Sulphuric acid solution with concentrations of 2%, 3%, 4%, 5%, and 6% have been prepared. 10 g of feed mixture is fed into the round bottom flask. Initially, 60 ml (2%) of H2SO4 solution is measured and then poured into round bottom flask. Now, the sample mixture is stirred thoroughly and placed in a heating mantel for different time intervals from half an hour to two hours. After completion of heating for every time interval, the sample is collected and filtered using filter paper. The filtrate is collected in specific gravity bottle and weighed. Readings of Specific Gravity (SG) bottle with filtrate for every sample is tabulated and their corresponding specific gravities have been calculated. Verification of specific gravities has been done using specific gravity vs weight MnSO4 of calibration chart. Similarly, the process is carried out for different H2SO4 concentrations (2 mol/L, 3 mol/L, 4 mol/L, 5 mol/L, 6 mol/L) and at different temperatures (40oC, 60oC, 80oC, 100oC, 110oC) and the readings have been tabulated (Tables 1, 2, 3, 4, and 5). Table 1 shows the % of Mn recovered with different H2SO4 concentrations (2 mol/L, 3 mol/L, 4 mol/L, 5 mol/L, 6 mol/L) at a temperature of 40oC. Table 2 shows the % of Mn recovered with different H2SO4 concentrations (2 mol/L, 3 mol/L, 4 mol/L, 5 mol/L, 6 mol/L) at a temperature of 60oC. Table 3 shows the % of Mn recovered with different H2SO4 concentrations (2 mol/L, 3 mol/L, 4 mol/L, 5 mol/L, 6 mol/L) at a temperature of 80oC. Table 4 shows the % of Mn recovered with different H2SO4 concentrations (2 mol/L, 3 mol/L, 4 mol/L, 5 mol/L, 6 mol/L) at a temperature of 100oC. Table 5 shows the % of Mn recovered with different H2SO4 concentrations (2 mol/L, 3 mol/L, 4 mol/L, 5 mol/L, 6 mol/L) at a temperature of 110oC.

Table 1. Temperature at 40oC
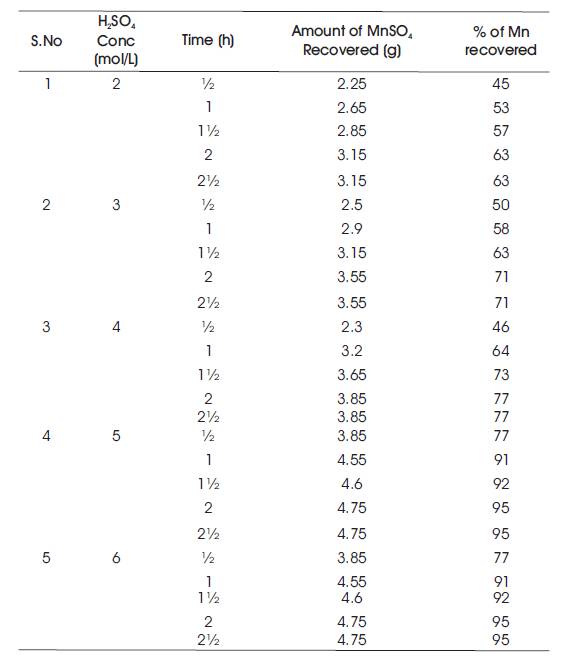
Table 2. Temperature at 60oC
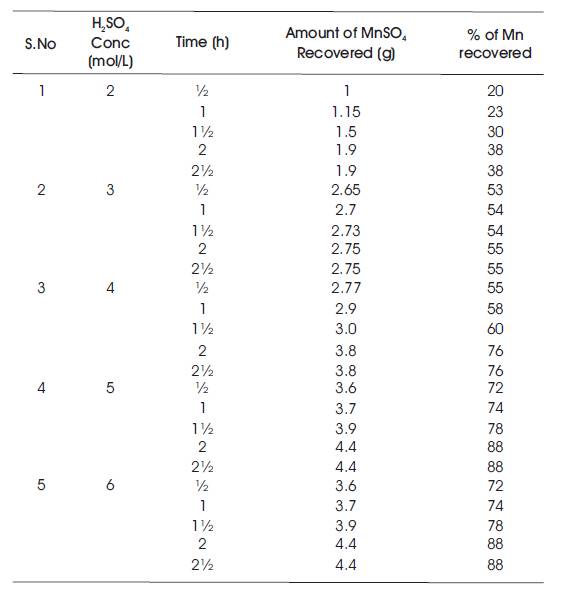
Table 3. Temperature at 80oC
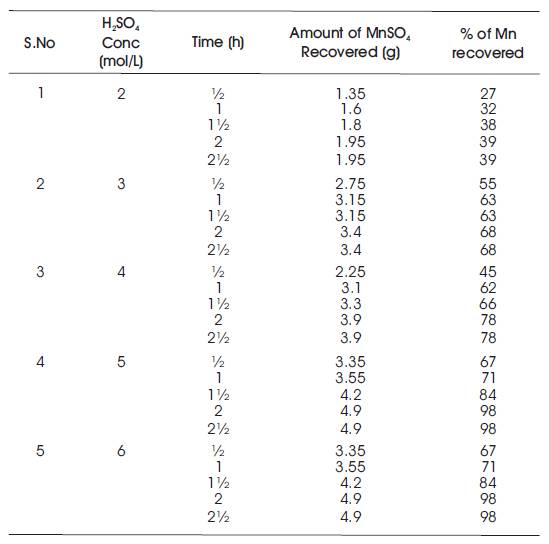
Table 4. Temperature at 100oC
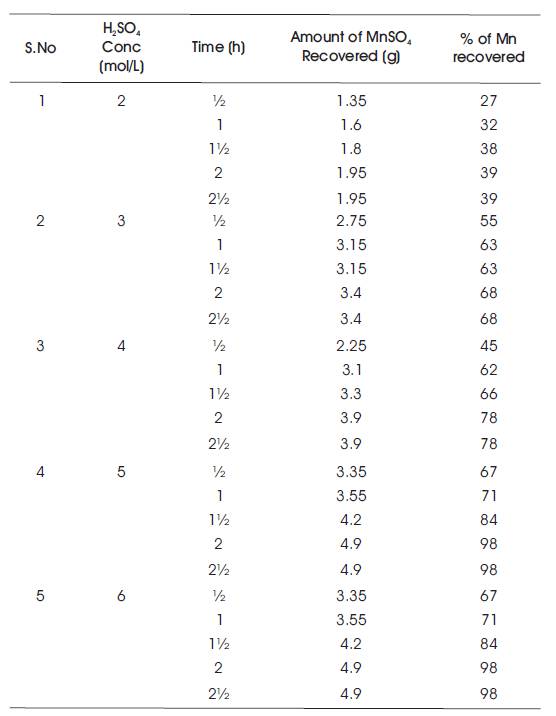
Table 5. Temperature at 110oC
Analysis was done using specific gravity method, wherein the experimental results were checked and the calibration curve is shown in Figure 1.
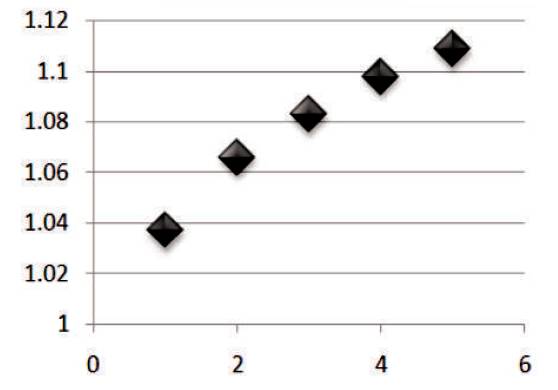
Figure 1. Calibration Curve (Graph between Specific Gravity and Weight of MnSO4 )
Analysis was done using standard titration with Ethylenediaminetetraacetic acid (EDTA) solution.
Manganese is a transition metal which forms colored compounds. In this experiment, the Mn2+ ions are converted to permanganate ions MnO4 -, which absorbs visible light and is dark purple in color. The conversion takes place by reaction of the manganese ion with persulphate ion, using silver ion as a catalyst.
The reaction is as follows:
2 MnO4- (aq) + 10 SO4-2 (aq) + 16 H+ (aq) 2 Mn2+ (aq) + 5 S2O8-2 (aq) + 8 H2O (aq)
Absorbance is a measure of the amount of light absorbed by a solution: the darker the solution, the higher the absorbance. Colored solutions selectively absorb some wavelengths of light, while allowing others to pass though. The permanganate appears purple because it allows red and blue light to pass, absorbing light in the green and yellow ranges.
Graphs are plotted between temperature vs % recovery of Mn for different H2SO4 concentrations (2% to 6%) and different time intervals (30 min to 150 min).
Figure 2 shows the graph between temperature vs % recovery of Mn for different H2SO4 concentration at ½ hour. From the graph, it is clear that as the temperature increases, the % recovery of Mn also increases. The highest % recovery of Mn is obtained for the sulfuric acid concentration of 5% & 6 %.
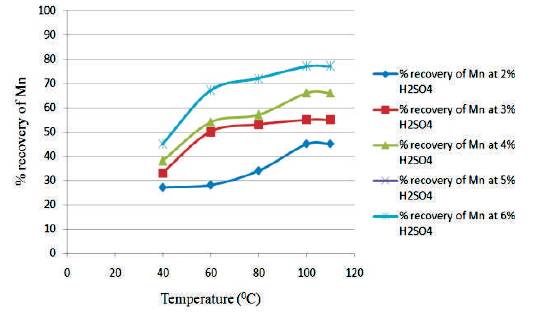
Figure 2. Graph between Temperature vs % Recovery of Mn for different H2SO4 Conc at ½ hour
Figure 3 shows the graph between temperature vs % recovery of Mn for different H2SO4 Conc. at 1 hour. From the graph, it is clear that as the temperature increases, the % recovery of Mn also increases. The highest % recovery of Mn is obtained for the sulfuric acid concentration of 5% & 6 %.
Figure 3. Graph between Temperature vs % Recovery of Mn for different H2SO4 Conc at 1hour
Figure 4 shows the graph between temperature vs % recovery of Mn for different H2SO4 Conc. at 1½ hour. From the graph, it is clear that as the temperature increases, the % recovery of Mn also increases. The highest % recovery of Mn is obtained for the sulfuric acid concentration of 5% & 6 %.
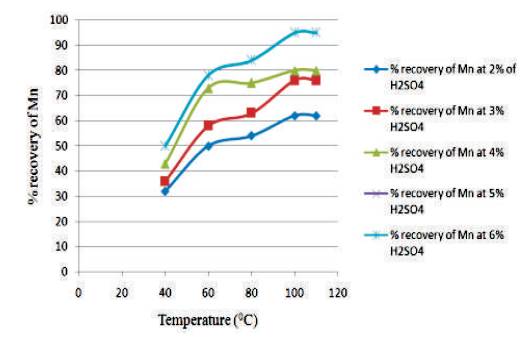
Figure 4. Graph between Temperature vs % Recovery of Mn for different H2SO4 Conc at 1½ hours
Figure 5 shows the graph between temperature vs % recovery of Mn for different H2SO4 Conc. at 2 hours. From the graph, it is clear that as the temperature increases, the % recovery of Mn also increases. The highest % recovery of Mn is obtained for the sulfuric acid concentration of 5% & 6 %.
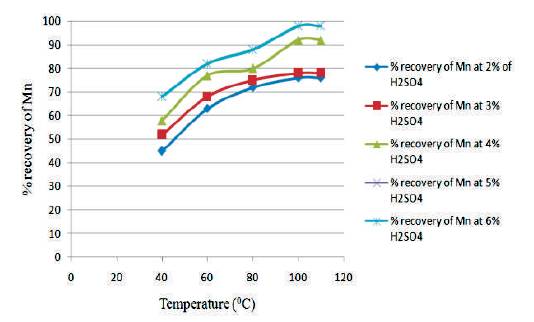
Figure 5. Graph between Temperature vs % Recovery of Mn for different H2SO4 Conc at 1½ hours
Figure 6 shows the graph between temperature vs % recovery of Mn for different H2SO4 Conc. at 2½ hours. From the graph, it is clear that as the temperature increases, the % recovery of Mn also increases. The highest % recovery of Mn is obtained for the sulfuric acid concentration of 5% & 6 %.
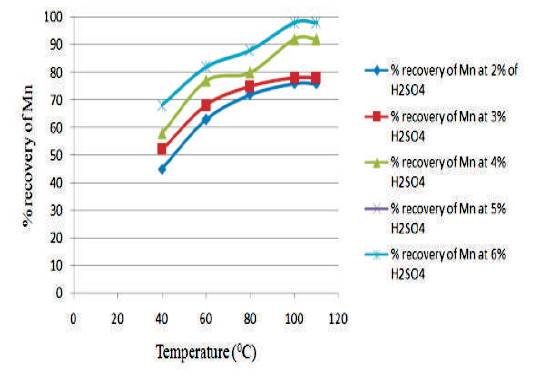
Figure 6. Graph between Temperature vs % Recovery of Mn for different H2SO4 Conc at 2½ hour
From all these graphs, the following are observed as results.
The present study evaluates the possibility of selective leaching of manganese from complex, in a high temperature sulphuric acid medium. The factors affecting the sulphuric acid leaching of the manganese complex in presence of various parameters were investigated. The three variables studied were temperature of leaching medium, leaching time, and concentration of sulphuric acid. It is observed that the leaching efficiency has increased remarkably as the concentration of sulfuric acid increases from 2% to 6%. The results show that temperature also has a profound effect on leaching efficiency and as temperature increases (from 40oC to 100oC), the leaching efficiency increases and after some point of temperature (100oC), it does not change with temperature. Thus, the optimum conditions established for maximum metal (manganese) extraction (98%) are: time 2 hours, temperature 100 °C & sulfuric acid concentration 5.0%.
This study evaluates the possibility of selective leaching of manganese from complex, in a high temperature sulphuric acid medium. The factors affecting the sulphuric acid leaching of the manganese complex in presence of various parameters were investigated. The three variables studied were temperature of leaching medium, leaching time, and concentration of sulphuric acid. Under optimum conditions, the recovery of Mn was 98%. The present process may find application of separation of manganese from iron and aluminium at high temperature during various hydrometallurgical treatment of manganese based ores.